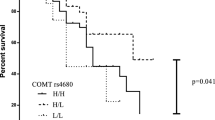
Abstract
Tardive dyskinesia (TD) in schizophrenia patients treated with antipsychotic medications and l-dopa induced dyskinesia (LID) among Parkinson's disease (PD) affected individuals share similar clinical features. Both conditions are induced by chronic exposure to drugs that target dopaminergic receptors (antagonists in TD and agonists in LID) and cause pulsatile and nonphysiological stimulation of these receptors. We hypothesized that the two motor adverse effects partially share genetic risk factors such that certain genetic variants exert a pleiotropic effect, influencing susceptibility to TD as well as to LID. In this pilot study, we focused on 21 TD-associated SNPs, previously reported in TD genome-wide association studies or in candidate gene studies. By applying logistic regression and controlling for relevant clinical risk factors, we studied the association of the SNPs with LID vulnerability in two independent pharmacogenetic samples. We included a Jewish Israeli sample of 203 PD patients treated with l-dopa for a minimum of 3 years and evaluated the existence or absence of LID (LID+ = 128; LID− = 75). An Italian sample was composed of early LID developers (within the first 3 years of treatment, N = 187) contrasted with non-early LID developers (after 7 years or more of treatment, N = 203). None of the studied SNPs were significantly associated with LID susceptibility in the two samples. Therefore, we were unable to obtain proof of concept for our initial hypothesis of an overlapping contribution of genetic risk factors to TD and LID. Further studies in larger samples are required to reach definitive conclusions.
Similar content being viewed by others
References
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458
Al Hadithy AF, Ivanova SA, Pechlivanoglou P et al (2009) Tardive dyskinesia and DRD3, HTR2Aand HTR2Cgene polymorphisms in Russian psychiatric inpatients from Siberia. Prog Neuropsychopharmacol Biol Psychiatry 33:475–481
Al Hadithy AF, Ivanova SA, Pechlivanoglou P et al (2010) Missense polymorphisms in three oxidative-stress enzymes (GSTP1, SOD2, and GPX1) and dyskinesias in Russian psychiatric inpatients from Siberia. Hum Psychopharmacol 25:84–91
Aubert I, Guigoni C, Håkansson K et al (2005) Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol 57:17–26
Bakker PR, van Harten PN, van Os J (2006) Antipsychotic-induced tardive dyskinesia and the Ser9Gly polymorphism in the DRD3 gene: a meta analysis. Schizophr Res 83:185–192
Bakker PR, van Harten PN, van Os J (2008) Antipsychotic-induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1A2 and MnSOD genes: a meta-analysis of pharmacogenetic interactions. Mol Psychiatry 13:544–556
Blanchet PJ (2003) Antipsychotic drug-induced movement disorders. Can J Neurol Sci 30:S101–S107
Boke O, Gunes S, Kara N et al (2007) Association of serotonin 2A receptor and lack of association of CYP1A2 gene polymorphism with tardive dyskinesia in a Turkish population. DNA Cell Biol 26:527–531
Crowley JJ, Sullivan PF, McLeod HL (2009) Pharmacogenomic genome-wide association studies: lessons learned thus far. Pharmacogenomics 10:161–163
de Lau LM, Verbaan D, Marinus J, Heutink P, van Hilten JJ (2012) Catechol-O-methyltransferase Val158Met and the risk of dyskinesias in Parkinson's disease. Mov Disord 27:132–135
Del Sorbo F, Albanese A (2008) Levodopa-induced dyskinesias and their management. J Neurol 255(Suppl 4):32–41
Delfs JM, Ellison GD, Mercugliano M, Chesselet MF (1995) Expression of glutamic acid decarboxylase mRNA in striatum and pallidum in an animal model of tardive dyskinesia. Exp Neurol 133:175–188
Egan MF, Apud J, Wyatt RJ (1997) Treatment of tardive dyskinesia. Schizophr Bull 23:583–609
Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG (2007) Levodopa-induced dyskinesias. Mov Disord 22:1379–1389
Fisone G, Bezard E (2011) Molecular mechanisms of l-dopa-induced dyskinesia. Int Rev Neurobiol 98:95–122
Foltynie T, Cheeran B, Williams-Gray CH et al (2009) BDNF val66met influences time to onset of levodopa induced dyskinesia in Parkinson's disease. J Neurol Neurosurg Psychiatry 80:141–144
Greenbaum L, Alkelai A, Rigbi A, Kohn Y, Lerer B (2010) Evidence for association of the GLI2 gene with tardive dyskinesia in patients with chronic schizophrenia. Mov Disord 25:2809–2817
Greenbaum L, Alkelai A, Zozulinsky P, Kohn Y, Lerer B (2012) Support for association of HSPG2 with tardive dyskinesia in Caucasian populations. Pharmacogenomics J 12:513–20
Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E (2007) Altered D(1) dopamine receptor trafficking in parkinsonian and dyskinetic non-human primates. Neurobiol Dis 2007(26):452–463
Haddad PM, Dursun SM (2008) Neurological complications of psychiatric drugs: clinical features and management. Hum Psychopharmacol 23(Suppl):15–26
Hassin-Baer S, Molchadski I, Cohen OS et al (2011) Gender effect on time to levodopa-induced dyskinesias. J Neurol 258:2048–2053
Hely MA, Morris JG, Reid WG, Trafficante R et al (2005) Sydney Multicenter Study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 20:190–199
Jenner P (2008) Molecular mechanisms of l-DOPA-induced dyskinesia. Nat Rev Neurosci 9:665–677
Kaiser R, Hofer A, Grapengiesser A et al (2003) l-dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology 60:1750–1755
Kane JM (1995) Tardive dyskinesia: epidemiological and clinical presentation. In: Bloom FE, Kupfer DJ (eds) Psychopharmacology: the 4th generation of progress. Raven, New York
Kang SG, Lee HJ, Choi JE, An H, Rhee M, Kim L (2009) Association study between glutathione S-transferase GST-M1, GST-T1, and GST-P1 polymorphisms and tardive dyskinesia. Hum Psychopharmacol 24:55–60
Lee HJ, Kang SG (2011) Genetics of tardive dyskinesia. Int Rev Neurobiol 98:231–264
Lerer B, Segman RH (2006) Pharmacogenetics of antipsychotic therapy: pivotal research issues and the prospects for clinical implementation. Dialogues Clin Neurosci 8:85–94
Lerer B, Segman RH, Fangerau H et al (2002) Pharmacogenetics of tardive dyskinesia: combined analysis of 780 patients supports association with dopamine D3 receptor gene Ser9Gly polymorphism. Neuropsychopharmacology 27:105–119
Lerer B, Segman RH, Tan EC et al (2005) Combined analysis of 635 patients confirms an age-related association of the serotonin 2A receptor gene with tardive dyskinesia and specificity for the non-orofacial subtype. Int J Neuropsychopharmacol 8(3):411–425
Lin JJ, Yueh KC, Lin SZ, Harn HJ, Liu JT (2007) Genetic polymorphism of the angiotensin converting enzyme and L-dopa-induced adverse effects in Parkinson's disease. J Neurol Sci 252:130–134
Linazasoro G (2005) New ideas on the origin of L-dopa-induced dyskinesias: age, genes and neural plasticity. Trends Pharmacol Sci 26:391–397
Margolese HC, Chouinard G, Kolivakis TT, Beauclair L, Miller R (2005) Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatry 50:541–547
Mercuri NB, Bernardi G (2005) The ‘magic’ of L-dopa: why is it the gold standard Parkinson's disease therapy? Trends Pharmacol Sci 26:341–344
Molchadski I, Korczyn AD, Cohen OS et al (2011) The role of apolipoprotein E polymorphisms in levodopa-induced dyskinesia. Acta Neurol Scand 123:117–121
Naidu PS, Singh A, Kulkarni SK (2002) Carvedilol attenuates neuroleptic-induced orofacial dyskinesia: possible antioxidant mechanisms. Br J Pharmacol 136:193–200
Olanow CW, Obeso JA, Stocchi F (2006) Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol 5:677–687
Oliveri RL, Annesi G, Zappia M et al (1999) Dopamine D2 receptor gene polymorphism and the risk of levodopa-induced dyskinesias in PD. Neurology 53:1425–1430
Paus S, Gadow F, Knapp M, Klein C, Klockgether T, Wüllner U (2009) Motor complications in patients form the German Competence Network on Parkinson's disease and the DRD3 Ser9Gly polymorphism. Mov Disord 24:1080–1084
Prashanth LK, Fox S, Meissner WG (2011) l-Dopa-induced dyskinesia-clinical presentation, genetics, and treatment. Int Rev Neurobiol 98:31–54
Purcell S, Neale B, Todd-Brown K et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575
Rascol O, Brooks DJ, Korczyn AD et al (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 342:1484–1491
Remington G (2007) Tardive dyskinesia: eliminated, forgotten, or overshadowed? Curr Opin Psychiatry 20:131–137
Sagara Y (1998) Induction of reactive oxygen species in neurons by haloperidol. J Neurochem 71:1002–1012
Sakai K, Gao XM, Hashimoto T, Tamminga CA (2001) Traditional and new antipsychotic drugs differentially alter neurotransmission markers in basal ganglia-thalamocortical neural pathways. Synapse 39:152–160
Segman RH, Heresco-Levy U, Finkel B et al (2000) Association between the serotonin 2C receptor gene and tardive dyskinesia in chronic schizophrenia: additive contribution of 5-HT2Cser and DRD3gly alleles to susceptibility. Psychopharmacology (Berl) 152:408–413
Segman RH, Heresco-Levy U, Finkel B et al (2001) Association between the serotonin 2A receptor gene and tardive dyskinesia in chronic schizophrenia. Mol Psychiatry 6:225–229
Sharma JC, Macnamara L, Hasoon M, Vassallo M, Ross I (2006) Cascade of levodopa dose and weight-related dyskinesia in Parkinson's disease (LD-WD-PD cascade). Parkinsonism Relat Disord 12:499–505
Sharma JC, Bachmann CG, Linazasoro G (2010) Classifying risk factors for dyskinesia in Parkinson's disease. Parkinsonism Relat Disord 16:490–497
Shulman JM, De Jager PL, Feany MB (2011) Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol 28:193–222
Strong JA, Dalvi A, Revilla FJ et al (2006) Genotype and smoking history affect risk of levodopa-induced dyskinesias in Parkinson's disease. Mov Disord 21:654–659
Syu A, Ishiguro H, Inada T et al (2010) Association of the HSPG2 gene with neuroleptic-induced tardive dyskinesia. Neuropsychopharmacology 35:1155–1164
Tanaka S, Syu A, Ishiguro H et al (2011) DPP6 as a candidate gene for neuroleptic-induced tardive dyskinesia. Pharmacogenomics J. doi:10.1038/tpj.2011.36
Tenback DE, van Harten PN (2011) Epidemiology and risk factors for (tardive) dyskinesia. Int Rev Neurobiol 98:211–230
Tenback DE, van Harten PN, van Os J (2009) Non-therapeutic risk factors for onset of tardive dyskinesia in schizophrenia: a meta-analysis. Mov Disord 24:2309–2315
van Harten PN, Tenback DE (2011) Tardive dyskinesia: clinical presentation and treatment. Int Rev Neurobiol 98:187–210
Watanabe M, Harada S, Nakamura T et al (2003) Association between catechol-O-methyltransferase gene polymorphisms and wearing-off and dyskinesia in Parkinson's disease. Neuropsychobiology 48:190–193
Zai CC, De Luca V, Hwang RW et al (2007) Meta-analysis of two dopamine D2 receptor gene polymorphisms with tardive dyskinesia in schizophrenia patients. Mol Psychiatry 12:794–795
Zappia M, Annesi G, Nicoletti G et al (2005) Sex differences in clinical and genetic determinants of levodopa peak-dose dyskinesias in Parkinson disease: an exploratory study. Arch Neurol 62:601–605
Acknowledgments
This study was supported in part by a Rapid Response Innovation grant from the Michael J. Fox Foundation for Parkinson's Research. The Italian DNA samples were from the “Parkinson Biobank” of the Parkinson Institute, Istituti Clinici di Perfezionamento, Italy (www.parkinsonbiobank.com). This biobank is part of the Telethon Genetic Biobank Network (http://www.biobanknetwork.org) supported by TELETHON Italy (project no. GTB07001) and by the “Fondazione Grigioni per il Morbo di Parkinson.”
Conflict of Interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 46 kb)
Rights and permissions
About this article
Cite this article
Greenbaum, L., Goldwurm, S., Zozulinsky, P. et al. Do Tardive Dyskinesia and l-Dopa Induced Dyskinesia Share Common Genetic Risk Factors? An Exploratory Study. J Mol Neurosci 51, 380–388 (2013). https://doi.org/10.1007/s12031-013-0020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-013-0020-x