Abstract
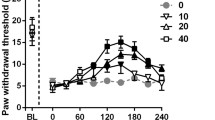
Neuropathic pain induced by sciatic nerve injury not only causes peripheral dysfunctions but also affects the cortical and subcortical regions of the brain. It is still unknown whether neuropathic pain could relate to behavioral and neurochemical alterations in the central nervous system. This paper deals with the effect of peripheral neuropathic pain on mechanical allodynia, neuropeptide levels, neuropeptide-degrading enzyme activities, and microglial cells in the brain regions of rats by applying chronic constriction injury, a partial sciatic nerve injury. We examined the possible protection effect on the allodynia and changes in levels of neuropeptides and microglial activation in chronic constriction injury of the rat brain by memantine. On 4 days after chronic constriction injury, the induction of mechanical allodynia was suppressed by memantine treatment. Reductions in the substance P in the hypothalamus and somatostatin in the periaqueductal gray of chronic constriction injury rat brain were reversed by memantine. This suggests the role of these neuropeptides in pain information processing in the brain. Immunohistochemical experiments revealed that the expression of CD11b, a marker protein of microglia, was increased in the hypothalamus and periaqueductal gray in the chronic constriction injury rat brain as compared with the controls, and memantine treatment could suppress the activation of microglia, suggesting the involvement of microglia in pain mechanism. The present behavioral, biochemical, and immunohistochemical studies demonstrated that peripheral neuropathic pain affects the neuropeptide levels and microglial activation in the brain regions, and these events described above may play an important role in neuropathic pain pathogenesis.







Similar content being viewed by others
Abbreviations
- AMC:
-
7-Amino-4-methylcoumarin
- anti-SOM:
-
Antisomatostatin
- anti-SP:
-
Antisubstance P
- BSA:
-
Bovine serum albumin
- CCI:
-
Chronic constriction injury
- CNS:
-
Central nervous system
- DAB:
-
3,3′-Diaminobenzidine
- EIA:
-
Enzyme immunoassay
- GABA:
-
γ-Aminobutyric acid
- HRP:
-
Horseradish peroxidase
- NMDA:
-
N-Methyl-d-aspartate
- PAG:
-
Periaqueductal gray
- PBS:
-
Phosphate-buffered saline
- PNS:
-
Peripheral nervous system
- POP:
-
Prolyl oligopeptidase
- SOM:
-
Somatostatin
- SOM-LI:
-
SOM-like immunoreactivity
- SP:
-
Substance P
- SP-LI:
-
SP-like immunoreactivity
- Suc-Gly-Pro-MCA:
-
Succinylglycyl-l-proline 4-methylcoumaryl-7-amide
References
Ahmed, M. M., Hoshino, H., Chikuma, T., Yamada, M., & Kato, T. (2004). Effect of memantine on the levels of glial cells, neuropeptides, and peptide-degrading enzymes in rat brain regions of ibotenic acid-treated Alzheimer’s disease model. Neuroscience, 126, 639–649.
Alzate, O., Hussain, S. R., Goettl, V. M., et al. (2004). Proteomic identification of brainstem cytosolic proteins in a neuropathic pain model. Molecular Brain Research, 128, 193–200.
Ambalavanar, R., Dessem, D., Moutanni, A., et al. (2006). Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience, 143, 875–884.
Auld, D. S., & Robitaille, R. (2003). Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron, 40, 389–400.
Bennett, G. J., & Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 33, 87–107.
Bianchi, M., Sotgui, M. L., Manfredi, B., & Sacerdote, P. (1996). Peripheral mononeuropathy affects hypothalamic and splenocyte beta-endorphin levels but not immune function in the rat. Brain Research Bulletin, 40, 269–272.
Brown, D. G., & Krupp, J. J. (2006). N-Methyl-d-aspartate receptor (NMDA) antagonists as potential pain therapeutics. Current Topics in Medicinal Chemistry, 6, 749–770.
Bucinskaite, V., Lundeberg, T., Stenfors, C., Belfrage, M., Hansson, P., & Theodorsson, E. (1995). Changes of neuropeptide concentrations in the brain following experimentally induced mononeuropathy in Wistar Kyoto and spontaneously hypertensive rats. Neuroscience Letters, 192, 93–96.
Carlton, S. M., & Hargett, G. L. (1995). Treatment with the NMDA antagonist memantine attenuates nociceptive responses to mechanical stimulation in neuropathic rats. Neuroscience Letters, 198, 115–118.
Caruso, C., Durand, D., Watanobe, H., & Lasaga, M. (2006). NMDA and group I metabotropic glutamate receptors activation modulates substance P release from the arcuate nucleus and median eminence. Neuroscience Letters, 393, 60–64.
Coyle, D. E. (2007). Spinal cord transcriptional profile analysis reveals protein trafficking and RNA processing as prominent processes regulated by tactile allodynia. Neuroscience, 144, 144–156.
Crack, P. J., Wu, T. J., Cummins, P. M., et al. (1999). The association of metalloendopeptidase EC 3.4.24.15 at the extracellular surface of the AtT-20 cell plasma membrane. Brain Research, 835, 113–124.
Dahms, P., & Mentlein, R. (1992). Purification of the main somatostatin-degrading protease from rat and pig brains, their action on other neuropeptides, and their identification as endopeptidases 24.15 and 24.16. European Journal of Biochemistry, 208, 145–154.
Davidson, E. M., & Carlton, S. M. (1998). Intraplantar injection of dextrorphan, ketamine, or memantine attenuates formalin-induced behaviors. Brain Research, 785, 136–142.
Dixon, W. J. (1980). Efficient analysis of experimental observations. Annual Review of Pharmacology and Toxicology, 20, 441–462.
Eisenberg, E., LaCross, S., & Strassman, A. M. (1995). The clinically tested N-methyl-d-aspartate receptor antagonist memantine blocks and reverses thermal hyperalgesia in a rat model of painful mono-neuropathy. Neuroscience Letters, 187, 17–20.
Ferrari, D., Chiozzi, P., Falzoni, S., et al. (1997). Extracellular ATP triggers IL-1β release by activation the purinergic P2Z receptor of human macrophages. The Journal of Immunology, 159, 1451–1458.
Finnerup, N. B., & Jensen, T. S. (2004). Spinal cord injury pain-mechanisms and treatment. European Journal of Neurology, 11, 73–82.
Greenamyre, J. T. (1986). The role of glutamate in neurotransmission and in neurologic disease. Archives of Neurology, 43, 1058–1063.
Hains, B. C., Yucra, J. A., & Hulsebosch, C. E. (2001). Reduction of pathological and behavioral deficits following spinal cord contusion injury with the selective cyclooxygenase-2 inhibitor NS-398. Journal of Neurotrauma, 18, 409–423.
Hains, B. C., Klein, J. P., Saab, C. Y., Craner, M. J., Black, J. A., & Waxman, S. G. (2003a). Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. The Journal of Neuroscience, 23, 8881–8892.
Hains, B. C., Willis, W. D., & Hulsebosch, C. E. (2003b). Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Experimental Brain Research, 149, 174–186.
Hartree, E. F. (1972). Determination of protein: a modification of the Lowry method that gives a linear photometric response. Analytical Biochemistry, 48, 422–427.
Helmchen, C., Fu, Q. G., & Sandkuhler, J. (1995). Inhibition of spinal nociceptive neurons by microinjections of somatostatin into the nucleus raphe magnus and the midbrain periaqueductal gray of the anesthetized cat. Neuroscience Letters, 187, 137–141.
Hua, X. Y., Chen, P., Marsala, M., & Yaksh, T. L. (1999). Intrathecal substance P-induced thermal hyperalgesia and spinal release of prostaglandin E2 and amino acids. Neuroscience, 89, 525–534.
Ji, R. R., Kohno, T., Moore, K. A., & Woolf, C. J. (2003). Central sensitization and LPT: Do pain and memory share similar mechanisms? Trends in Neurosciences, 26, 696–705.
Kato, T. (2004). Memantine: a therapeutic drug for Alzheimer’s disease and the comparison with MK-801. Folia Pharmacologica Japonica, 124, 145–151.
Kato, T., Nakano, T., Kojima, K., Nagatsu, T., & Sakakibara, S. (1980). Changes in prolyl endopeptidase during maturation of rat brain and hydrolysis of substance P by the purified enzymes. Journal of Neurochemistry, 35, 527–535.
Kreutzberg, G. W. (1996). Microglia: A sensor for pathological events in the CNS. Trends in Neurosciences, 19, 312–318.
Mantyh, P. W., & Hunt, S. P. (2004). Setting the tone: Superficial dorsal horn projection neurons regulate pain sensitivity. Trends in Neurosciences, 27, 582–584.
Medvedev, I. O., Malyshkin, A. A., Belozertseva, I. V., et al. (2004). Effects of low-affinity NMDA receptor channel blockers in two rat models of chronic pain. Neuropharmacology, 47, 175–183.
Milligan, E. D., Zapata, V., Chacur, M., et al. (2004). Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. European Journal of Neuroscience, 20, 2294–2302.
Minami, T., Okuda-Ashitaka, E., Hori, Y., et al. (1999). Involvement of primary afferent C-fibers in touch-evoked pain (allodynia) induced by prostaglandin E2. European Journal of Neuroscience, 11, 1849–1856.
Obata, H., Eisenach, J. C., Hussain, H., Bynum, T., & Vincler, M. (2006). Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. The Journal of Pain, 7, 816–822.
Onley, J. W., Labruyere, J., Wang, G., Wozniak, D. F., Price, M. T., & Sesma, M. A. (1991). NMDA antagonist neurotoxicity: Mechanism and prevention. Science, 254, 1515–1518.
Panerai, A. E., Sacerdote, P., Brini, A., Bianchi, M., & Mantegazza, P. (1988). Central nervous system neuropeptides after peripheral nerve deafferentiation. Peptides, 9, 319–324.
Paxinos, G., & Watson, C. (1986). The rat brain in stereotaxic coordinates (2nd ed.). San Diego: Academic.
Petrenko, A. B., Yamakura, T., Baba, H., & Shimoji, K. (2003). The role of N-methyl-d-aspartate (NMDA) receptors in pain: A review. Anesthesia & Analgesia, 97, 1108–1116.
Raghavendra, V., Tanga, F., & DeLeo, J. A. (2003a). Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. The Journal of Pharmacology and Experimental Therapeutics, 306, 624–630.
Raghavendra, V., Tanga, F., Rutkowski, M. D., & DeLeo, J. A. (2003b). Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain, 104, 655–664.
Restuccia, D., Di Lazzaro, V., Valeriani, M., Tonali, P., & Mauguiere, F. (1992). Segmental dysfunction of the cervical cord revealed by abnormalities of the spinal N13 potential in cervical spondylotic myelopathy. Neurology, 42, 1054–1063.
Rosen, A., Zhang, Y. X., Lund, I., Lundeberg, T., & Yu, L. C. (2004). Substance P microinjected into the periaqueductal gray matter induces antinociception and is released following morphine administration. Brain Research, 1001, 87–94.
Saito, T., Iwata, N., Tsubuki, S., et al. (2005). Somatostatin regulates brain amyloid βpeptide Aβ42 through modulation of proteolytic degradation. Nature Medicine, 11, 434–439.
Samad, T. A., Moore, K. A., Sapirstein, A., et al. (2001). Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature, 410, 471–475.
Seltzer, Z., Dubner, R., & Shir, Y. (1990). A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain, 43, 205–218.
Sotgui, M. L. (1993). Descending influence on dorsal horn neuronal hyperactivity in a rat model of neuropathic pain. Neuroreport, 4, 21–24.
Svensson, C. I., Hua, X. Y., Protter, A. A., Powell, H. C., & Yaksh, T. L. (2003). Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE2 release and thermal hyperalgesia. Neuroreport, 14, 1153–1157.
Sweitzer, S. M., Colburn, R. W., Rutkowski, M., & DeLeo, J. A. (1999). Acute peripheral inflammation induces moderate glial activation and spinal IL-1β expression that correlates with pain behavior in the rat. Brain Research, 829, 209–221.
Takeda, K., Uchiumi, F., Takita, M., & Kato, T. (1989). A rapid enzyme immunoassay for cholecystokinin octapeptide sulfate. Neurochemistry International, 15, 55–60.
Tseng, S. H. (1998). Suppression of autotomy by N-methyl-d-aspartate receptor antagonist (MK-801) in the rat. Neuroscience Letters, 240, 17–20.
Ueda, H., & Inoue, M. (2001). Animal models and peripheral nociception tests for the study of neuropathic pain. Folia Pharmacologica Japonica, 118, 89–95.
Wall, J. T., Xu, J., & Wang, X. (2002). Human brain plasticity: An emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Research Reviews, 39, 181–215.
Watkins, L. R., Milligan, E. D., & Maier, S. F. (2001). Glial activation: A driving force for pathological pain. Trends in Neurosciences, 24, 450–455.
Waxman, S. G., & Hains, B. C. (2006). Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends in Neurosciences, 29, 207–215.
Wilson, J. A., Garry, E. M., Anderson, H. A., et al. (2005). NMDA receptor antagonist treatment at the time of nerve injury prevents injury-induced changes in spinal NR1 and NR2B subunit expression and increases the sensitivity of residual pain behaviours to subsequently administered NMDA receptor antagonists. Pain, 117, 421–432.
Woolf, C. J., & Salter, M. W. (2000). Neuronal plasticity: Increasing the gain in pain. Science, 288, 1765–1768.
Acknowledgments
We are very grateful to Dr. Akira Tanaka for his valuable and helpful advice on the experiments and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeda, K., Muramatsu, M., Chikuma, T. et al. Effect of Memantine on the Levels of Neuropeptides and Microglial Cells in the Brain Regions of Rats with Neuropathic Pain. J Mol Neurosci 39, 380–390 (2009). https://doi.org/10.1007/s12031-009-9224-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-009-9224-5