Abstract
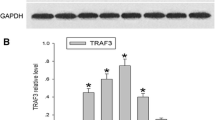
The adaptor protein, carboxy-terminal PDZ ligand of nNOS (CAPON), regulates the distribution of neuronal nitric oxide synthase (nNOS) that increased after spinal cord injury (SCI) and produces the key signaling molecule nitric oxide (NO). But little is known about the role of CAPON in the pathological process of SCI. The main objective of the present study was to investigate expression of CAPON and nNOS in a spinal cord contusion model in adult rats. Real time-polymerase chain reaction (PCR) and Western blot analysis revealed that mRNA and protein for CAPON increased at 2 h after SCI and reached the peak at 8 h, gradually recovered to the baseline level at 14 days. The expression of nNOS mRNA and protein was similar to that of CAPON. During the peak expression, CAPON mRNA was found in the ventral horn, mediate zone, dorsal horn, and white matter by in situ hybridization. Immunofluorescence showed that CAPON was colocalized with nNOS in neurons, oligodendrocytes, and some astrocytes of spinal cord tissues within 5 mm from the epicenter. Interaction between CAPON and nNOS was also detected by co-immunoprecipitation. Thus, the transient expression of high levels of CAPON may provide new insight into the secondary response after SCI.




Similar content being viewed by others
References
Akiko, H.-O., Okumura, N., Iwamatsui, A., Buijs, R. M., Romijni, H. J., & Nagai, K. (1999). Interaction of neuronal nitric-oxide synthase with a1-syntrophin in rat brain. Journal of Biological Chemistry, 274, 11736–11741.
Araque, A., Parpura, V., Sanzgiri, R. P., & Haydon, P. G. (1999). Tripartite synapses: Glia, the unacknowledged partner. Trends in Neurosciences, 22, 208–215.
Arundine, M., & Tymianski, M. (2003). Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium, 34(4–5), 325–137.
Bin, X., Wratten, N., Charych, E. I., Buyske, S., Firestein, B. L., & Brzustowicz, L. M. (2005). Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med, 2(10), e263.
Bracken, M. B., Freeman Jr., D. H., & Hellenbrand, K. (1981). Incidence of acute traumatic hospitalized spinal cord injury in the United States, 1970–1977. American Journal of Epidemiology, 113, 615–622.
Bredt, D. S., Hwang, P. M., Glatt, C. E., Lowenstein, C., Reed, R. R., & Snyder, S. H. (1991). Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature, 351, 714–718.
Bredt, D. S., & Snyder, S. H. (1992). Nitric oxide, a novel neuronal messenger. Neuron, 8, 3–11.
Brenman, J. E., Chao, D. S., Gee, S. H., McGee, A. W., Craven, S. E., Santillano, D. R., et al. (1996). Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell, 84, 757–767.
Brzustowicz, L. M., Simone, J., Mohseni, P., Hayter, J. E., Hodgkinson, K. A., et al. (2004). Linkage disequilibrium mapping of schizophrenia susceptibility to the CAPON region of chromosome 1q22. American Journal of Human Genetics, 74, 1057–1063.
Carroll, R. C., & Zukin, R. S. (2002). NMDA-receptor trafficking and targeting: Implications for synaptic transmission and plasticity. Trends in Neurosciences, 25, 571–577.
Cha, C. I., Kim, J. M., Shin, D. H., Kim, Y. S., Kim, J., Gurney, M. E., et al. (1998). Reactive astrocytes express nitric oxide synthase in the spinal cord of transgenic mice expressing a human Cu/Zn SOD mutation. Neuroreport, 9, 1503–1506.
Che, Y. H., Tamatani, M., & Tohyama, M. (2000). Changes in mRNA for post-synaptic density-95 (PSD-95) and carboxy-terminal PDZ ligand of neuronal nitric oxide synthase following facial nerve transection. Brain Res Mol Brain Res, 76, 325–335.
Cho, K. O., Hunt, C. A., & Kennedy, M. B. (1992). The rat brain post synaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron, 9, 929–942.
Christopherson, K. S., Hillier, B. J., Lim, W. A., & Bredt, D. S. (1999). PSD-95 assembles a ternary complex with the N-methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. Journal of Biological Chemistry, 274, 27467–27473.
Crowe, M. J., Bresnahan, J. C., Shuman, S. L., Masters, J. N., & Beattie, M. S. (1997). Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Natural Medicines, 3, 73–76.
David, L., Taconis, M., Maña, P., Correcha, M., Fordham, S., Staykova, M., et al. (2006). Neuronal nitric oxide synthase plays a key role in CNS demyelination. Journal of Neuroscience, 26, 12672–12681.
Dawson, V. L., Kizushi, V. M., Huang, P. L., Snyder, S. H., & Dawson, T. M. (1996). Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. Journal of Neuroscience, 16, 2479–2487.
Diaz-Ruiz, A., Ibarra, A., Perez-Severiano, F., Guizar-Sahagun, G., Grijalva, I., & Rios, C. (2002). Constitutive and inducible nitric oxide synthase activities after spinal cord contusion in rats. Neuroscience Letters, 319(3), 129–132.
Diaz-Ruiz, A., Vergara, P., Perez-Severiano, F., Segovia, J., Guizar-Sahagun, G., Ibarra, A., et al. (2005). Cyclosporin-A inhibits constitutive nitric oxide synthase activity and neuronal and endothelial nitric oxide synthase expressions after spinal cord injury in rats. Neurochemical Research, 30(2), 245–251.
Dumont, R. J., Okonkwo, D. O., Verma, S., Hurlbert, R. J., Boulos, P. T., Ellegala, D. B., et al. (2001). Acute spinal cord injury, Part I: Pathophysiologic mechanisms. Clinical Neuropharmacology, 24, 254–264.
Dusart, I., & Schwab, M. E. (1993). Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. European Journal of Neuroscience, 6, 712–724.
Fang, M., Jaffrey, S. R., Sawa, A., Ye, K., Luo, X., & Snyder, S. H. (2000). Dexras1: A G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron, 28, 183–193.
Fukaya, M., & Watanabe, M. (2000). Improved immunohistochemical detection of postsynaptically located PSD-95/SAP90 protein family by protease section pretreatment: A study in the adult mouse brain. Journal of Comparative Neurology, 426, 572–586.
Gary, M. R., & Bonnie, L. F. (2001). Binding of neuronal nitric-oxide synthase (nNOS) to carboxyl-terminal-binding protein (CtBP) changes the localization of CtBP from the nucleus to the cytosol. Journal of Biological Chemistry, 276, 48262–48268.
Genovese, T., Mazzon, E., Mariotto, S., Menegazzi, M., Cardali, S., Conti, A., et al. (2006). Modulation of nitric oxide homeostasis in a mouse model of spinal cord injury. Journal of Neurosurgery. Spine, 4(2), 145–153.
Griffith, O. W., & Stuehr, D. J. (1995). Nitric oxide synthases: Properties and catalytic mechanism. Annual Review of Physiology, 7, 707–736.
Gruner, J. A. (1992). A monitored contusion model of spinal cord injury in the rat. Journal of Neurotherapy, 9, 123–128.
Gruner, H. S., Lee, G., John, S. W., Maeda, N., & Smithies, O. (2002). Molecular phenotyping for analyzing subtle genetic effects in mice: Application to an angiotensinogen gene titration. Proceedings of the National Academy of Sciences of the United States of America, 99, 4602–4607.
Huang, Z., Huang, P. L., Panahian, N., Dalkara, T., Fishman, M. C., & Moskowitz, M. A. (1994). Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science, 265, 1883–1885.
Imai, Y., & Kohsaka, S. (2002). Intracellular signaling in M-CSF-induced microglia activation: Role of Iba1. Glia, 40, 164–174.
Jaffrey, S. R., Snowman, A. M., Eliasson, M. J., Cohen, N. A., & Snyder, S. H. (1998). CAPON: A protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron, 20, 115–124.
Jill, R. F., Herrmann, J. E., Woo, M. J., Tansey, K. E., Doan, N. B., & Sofroniew, M. V. (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. Journal of Neuroscience, 24(9), 2143–2155.
Kemppainen, R. J., & Behrend, E. N. (1998). Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. Journal of Biological Chemistry, 273, 3129–3131.
Kornau, H. C., Schenker, L. T., Kennedy, M. B., & Seeburg, P. H. (1995). Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science, 269, 1737–1740.
Kugler, P., & Drenckhahn, D. (1996). Astrocytes and Bergmann glia as an important site of nitric oxide synthase I. Glia, 16, 165–173.
Laurent, Se’ G., Grisoni, K., Archer, J., Vargas, C., Bertrand, A., & Anderson, J. E. (2005). CAPON expression in skeletal muscle is regulated by position, repair, NOS activity, and dystrophy. Experimental Cell Research, 302, 170–179.
Li, G. L., Farooque, M., & Holtz, A. (1999). Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathologica, 98, 473–480.
Lin, C. (2002). Nitric oxide synthase gene expression in injured spinal cord tissue. Chinese Medical Journal, 115(5), 740–742.
Liu, D., Bao, F., Prough, D. S., & Dewitt, D. S. (2005). Peroxynitrite generated at the level produced by spinal cord injury induces peroxidation of membrane phospholipids in normal rat cord: Reduction by a metalloporphyrin. Journal of Neurotrauma, 22(10), 1123–1133.
Liu, X. Z., Xu, X. M., Hu, R., et al. (1997). Neuronal and glial apoptosis after traumatic spinal cord injury. Journal of Neuroscience, 17, 5395–5406.
Martin, L. J., Chen, K., & Liu, Z. (2005). Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. Journal of Neuroscience, 25, 6449–6459.
Matsuyama, Y., Sato, K., Kamiya, M., Yano, J., Iwata, H., & Isobe, K. I. (1998). Nitric oxide: A possible etiologic factor in spinal cord cavitation. Journal of Spinal Disorders, 11, 248–252.
McDonald, D. R., Brunden, K. R., & Landreth, G. E. (1997). Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. Journal of Neuroscience, 17, 2284–2294.
McGeer, P. L., Itagaki, S., Boyes, B. E., & McGeer, E. G. (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology, 38, 1285–1291.
Miscusi, M. (2002). The role of constitutive nitric oxide synthase in pathogenesis of secondary lesion after spinal cord injury preliminary result. Journal of Neurosurgical Sciences, 46(2), 55–59.
Murphy, S. (2000). Production of nitric oxide by glial cells: Regulation and potential roles in the CNS. Glia, 29, 1–13.
Nianzhen, L., Sul, J.-Y., & Haydon, P. G. (2003). A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. Journal of Neuroscience, 23(32), 10302–10310.
Perry, V. H., Brown, M. C., & Lunn, E. R. (1991). Very slow retrograde and Wallerian degeneration in the CNS of C57BL/Ola mice. European Journal of Neuroscience, 3, 102–105.
Ponting, C. P., & Phillips, C. (1995). DHR domains in syntrophins, neuronal NO synthases and other intracellular proteins. Trends in Biochemical Sciences, 20, 102–103.
Rameau, G. A., Chiu, L. Y., & Ziff, E. B. (2004). Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-Methyl-d-aspartate receptor. Journal of Biological Chemistry, 279, 14307–14314.
Saito, S., Kidd, G. J., Trapp, B. D., Dawson, T. M., Bredt, D. S., Wilson, D. A., et al. (1994). Rat spinal cord neurons contain nitric oxide synthase. Neuroscience, 59, 447–456.
Samie, R., Benfenati, J. F., Snowman, A. M., Czernik, A. J., & Snyder, S. H. (2001). Neuronal nitric-oxide synthase localization mediated by a ternary complex with synapsin and CAPON. PNAS, 5, 3199–3204.
Sharma, H. S., Badgaiyan, R. D., Alm, P., Mohanty, S., & Wiklund, L. (2005). Neuroprotective effects of nitric oxide synthase inhibitors in spinal cord injury-induced pathophysiology and motor functions: An experimental study in the rat. Annals of the New York Academy of Sciences, 1053, 422–434.
Vaziri, N. D., Lee, Y. S., Lin, C. Y., Lin, V. W., & Sindhu, R. K. (2004). NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Research, 995(1), 76–83.
Volterra, A., Magistretti, P. J., & Haydon, P. G. (2002). The tripartite synapse: Glia in synaptic transmission. Oxford: Oxford UP.
Xia, Y., Berlowitz, C. O., & Zweier, J. L. (2006). PIN inhibits nitric oxide and superoxide production from purified neuronal nitric oxide synthase. Biochimica et Biophysica Acta, 1760(9), 1445–1449.
Xiong, Y., Rabchevsky, A. G., & Hall, E. D. (2007). Role of peroxynitrite in secondary oxidative damage after spinal cord injury. Journal of Neurochemistry, 100(3), 639–649.
Yong, C., Arnold, P. M., Zoubine, M. N., et al. (1998). Apoptosis in cellular compartments of rat spinal cord after severe contusion injury. Journal of Neurotrauma, 15, 459–472.
Young, W. (1993). Secondary injury mechanisms in acute spinal cord injury. Journal of Emergency Medicine, 11, 13–22.
Zheng, Y., Li, H., Qin, W., Chen, W., Duan, Y., et al. (2005). Association of the carboxyl-terminal PDZ ligand of neuronal nitric oxide synthase gene with schizophrenia in the Chinese Han population. Biochemical and Biophysical Research Communications, 328, 809–815.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30300099), Natural Science Foundation of Jiangsu province (No. BK2003035, No. BK2006547), and “Six Talent Peak” Foundation of Jiangsu province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chun Cheng and Xin Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cheng, C., Li, X., Gao, S. et al. Expression of CAPON after Spinal Cord Injury in Rats. J Mol Neurosci 34, 109–119 (2008). https://doi.org/10.1007/s12031-007-9019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-007-9019-5