Abstract
Purpose
Among all forms of cancers, hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide. There are several treatment options for HCC ranging from loco-regional therapy to surgical treatment. Yet, there is high morbidity and mortality. Recent research focus has shifted towards more effective and less toxic cancer treatment options. Curcumin, the active ingredient in the Curcuma longa plant, has gained widespread attention in recent years because of its multifunctional properties as an antioxidant, anti-inflammatory, antimicrobial, and anticancer agent.
Methods
A systematic search of PubMed, Embase and Google Scholar was performed for studies reporting incidence of HCC, risk factors associated with cirrhosis and experimental use of curcumin as an anti-cancer agent.
Results
This review exclusively encompasses the anti-cancer properties of curcumin in HCC globally and it’s postulated molecular targets of curcumin when used against liver cancers.
Conclusions
This review is concluded by presenting the current challenges and future perspectives of novel plant extracts derived from C. longa and the treatment options against cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a primary tumor of the liver and ranks among the fifth most common cancers globally. It is the third leading cause of cancer-related death after lung, colorectal, and stomach cancers [1]. The HCC includes 75–85% of all primary liver cancers and is a significant concern worldwide as it kills half a million people annually around the world [2,3,4]. In 2021, 42,230 HCC-related cases were recorded worldwide that showed 29,890 in men and 12,340 in women [5]. Research has shown that sub-Saharan Africa and Eastern Asia with Magnolia demonstrate the highest age-standardized incidence rates (ASIR) for HCC (more than 20 per 100,000 individuals). In contrast, South Central Asia, followed by Central and Eastern Europe and Western Asia (less than 5 per 100,000 people) [6,7,8]. The HCC incidence has increased from 3.1 to 5.1 per 100,000 people in the United States (US) between 1996 and 2006, while liver cancer mortality increased from 3.3 to 4.0 per 100,000 people [9]. The HCC rate has escalated annually by 2–3%, primarily due to factors including cirrhosis, hepatitis C virus (HCV), and non-alcoholic steatohepatitis (NASH) [10, 11]. The developmental pathway of HCC is described in Fig. 1.
Among the many factors, chronic hepatitis virus (HBV) is responsible for over 50% of all global cases [12]. Apart from conventional chemotherapy, several curative protocols such as liver transplantation and surgical resection are used to treat HCC [13]. However, owing to late diagnosis and poor response rates, these surgical and systemic treatments are often unsuccessful in providing survival benefits and improving mortality rates [14]. Up to 80% of patients experiencing HCC recurrence were observed within 5 years [15]. As a result, developing successful and safe therapeutics to increase the effectiveness of HCC therapy is critical. Unfortunately, current therapies have not been proven to be devoid of side effects during prolonged treatments [16]. Side effects, including but not limited to gastrointestinal toxicity, hepatotoxicity, cardiotoxicity, and neurotoxicity, are widespread among patients undergoing chemotherapies and radiotherapies against cancers, including HCC [17]. Therefore, the ongoing quest for anticancer agents derived from plants has played a crucial role in determining how to make chemotherapy safer and reduce its repercussions.
Traditional medicine has contributed many novel therapeutic compounds for preventive and curative treatment compared to other drug discovery sources. Over several decades, scientists analyzed nearly 200 combinations of different anticancer drugs. Half of these drugs come from natural products and their derivatives, which are safer and biologically advantageous [18, 19]. In addition, due to their structural diversification, secondary metabolites like flavonoids, terpenes, vitamins, oils, among others, have been proven to possess antimutagenic and anticancer properties [20, 21]. Among these, the natural phenolic compound has curcumin has anti-inflammatory properties that make it an ideal candidate for treating such chronic diseases [22]. Multiple tumor studies have displayed chemopreventive evidence of this potential therapeutic agent on HCC in human hepatoma cells. Different pathways have been studied that show the mechanism of action of curcumin, which includes prevention of tumor division, metastasis, and triggering apoptosis [23,24,25]. However, its low bioavailability and short lifetime in the human body is a significant concern. Studies have been done to functionalize curcumin with nanomaterials and other formulations like micelles, microspheres, emulsions, liposomes, and nanogels. In a recent study by Matias and colleagues, it was observed that phytosomes have a considerable potential to be used along with natural drugs, like curcumin, to improve their biocompatibility in the body and therefore effectively aid in the treatment of many inflammatory disease conditions, including cancers [26, 27].
Statistical Aspects of Hepatocellular Carcinoma Across the World in Particular India, USA, and GCC
Hepatocellular carcinoma has an annual fatality ratio (mortality-to-incidence ratio) of 0.92, the highest reported for a human cancer worldwide [1, 28]. In most countries, men have 2 to 3 times more HCC incidence and mortality than women. The most considerable incidence rates are found in underdeveloped nations [29]. However, this type of cancer is rampant in Eastern Asia, South-East Asia, Northern Africa, and Southern Africa [30]. Liver cancer is among the main reasons for death in Mongolia, Thailand, Cambodia, Egypt, and Guatemala. HCC is caused by chronic infection with the hepatitis B virus (HBV) or the hepatitis C virus (HCV), aflatoxin-polluted foods, excessive alcohol consumption, smoking, type 2 diabetes, and obesity [31]. Geographically, the critical risk factors differ significantly. Fatal HBV infection, aflatoxin exposure, or both are the critical determinants in high-risk HCC infection areas include China, the Republic of Korea, and sub-Saharan Africa [32]. Table 1 shows the number of new liver cancer cases and the estimated deaths in different global regions.
However, HCV infection may be the primary reason in other nations like Japan, Italy, and Egypt. Since the late 1970s, rates and mortality of HCC have decreased in many high-risk countries in Eastern and South-Eastern Asia and Japan. Since 1995, Italy’s rates have also been reduced [6, 33]. These patterns are most likely due to lower HBV and HCV seroprevalence in the population and lower aflatoxin exposure [34]. In 2015, it was reported that 290 million people worldwide had chronic infections that were usually asymptomatic and that many infected people went undiagnosed [35]. In high-risk countries like East Asia, the possible effect of HBV vaccination has been a significant public health achievement and thus has successfully helped reduce the number of HBV infection cases and HCC cases [36]. Even though non-viral risk factors also contribute to liver cancers, eliminating viral hepatitis remains the most crucial strategy for preventing HCC cases worldwide. HBV infection accounts for 56% and HCV infection for 20% of liver cancer deaths, respectively [30]. This can be understood from Fig. 2, which reveals the global trend in the number of HCC cases in 2018.
Trends in HCC cases across different global regions in 2018. a The pie chart shows the distribution of HCC for both sexes. b The pie chart shows the mortality cases primarily due to HCC. Data were taken with permission from [36], copyright@2019 (WCRJ)
By the end of 2019, compulsory HBV vaccination was launched for babies among 189 member states, with about 85% of infants receiving three vaccine doses worldwide [37]. However, the countries with the maximum rates of HCV infection are primarily low and middle-income countries, where most instances of diseases occur because of unhygienic injections and other inappropriate methods [38]. Therefore, a key element of HCV management is increasing safety initiatives to control infection, such as transfusion screening, precautions in transmittance through intimate relations like a mother to child, the availability of sterile needles, and control of infections in health care utilities [38].
India
There is a lack of nationally representative data, so we must depend on autopsy studies, national cancer registries, and population-based surveillance data to estimate the frequency of [39]. In India, the incidence rate of HCC based on age varies from 0.7 to 7.5 per 100,000 people per year for males and 0.2 to 2.2 per 100,000 people per year for women. In India, the female to male ratio for HCC is 1:4, and the average age group with HCC is 40–70 years [40]. The age-dependent death rate for HCC in India is 6.8/100,000 for males and 5.1/100,000 for women [39]. Cirrhosis is found in 70–97% of HCC cases and is one of the most prominent risk factors that cause HCC globally [41]. The World Gastroenterology Organization lists the following risk factors for HCC: chronic hepatitis B or C, obesity (NAFLD), aflatoxin (cofactor with HBV), tobacco, tyrosinosis, hemochromatosis, alcoholic and primary biliary cirrhosis, a1-antitrypsin deficiency, autoimmune chronic active hepatitis, diabetes, and viral load [42]. It was also noted that males had more chances of developing HCC as compared to females.
The occurrence of HCC among patients with cirrhosis in India is 1.6% each year during a 563-person-year follow-up period [41]. Furthermore, the incidence of HBV infection in non-tribal and tribal populations was 2.4% and 15.9%, respectively, in a comprehensive analysis of 54 Indian studies [43]. As a result, the yearly number of HCC cases related to HBV is predicted to be between 25 and 35,000. In addition, many HCC patients may be HBeAg-positive, indicating a reduced HBV DNA burden that is linked to a decreased risk of HCC. Unpublished statistics from multiple tertiary health centers in India indicate that the prevalence of HCC is rising. From 1990 to 2012, 1062 individuals with confirmed diagnoses were reported at the All India Institute of Medical Sciences (AIIMS) in New Delhi’s liver clinic. Figure 3 discusses this trend in the number of HCC cases from 1990 to 2012.
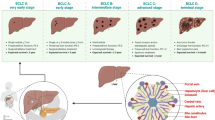
Trends in HCC cases in India from 1990 to 2012. a The graph represents the year-wise distribution of patients with HCC in the country. b Graphical representation of etiology of HCC in India. c Pie chart representing the age distribution of patients with HCC. d BCLC staging of HCC. The figure depicts the Barcelona Clinic Liver Cancer (BCLC) staging and indicates that more than 50% of HCC patients of presentation have BCLC-A and B staging, which were offered treatment were as an equal proportion of patients had advanced stage (BCLC-C and D). Data were taken with permission from ref. [40], copyright@2014 (JCEH)
Cirrhosis of the liver caused by HBV, HCV, excessive alcoholic intake, exposure to aflatoxin, and NAFLD are significant risk factors that cause HCC in India. According to reports from tertiary healthcare institutions in India, 70–97% of patients with HCC showed liver cirrhosis when diagnosed. On the other hand, reports from Europe and the US found that the yearly occurrence of HCC in HB20, HCV, or alcohol-induced cirrhosis varied from 2.2%, 3.8%, and 1.7%, respectively [44, 45]. Also, the incidence of HCC is more rampant in East Asian nations [46,47,48]. Although India has roughly 40–45 million HBV carriers and 10 million HCV-infected persons, cancer registries through the Indian Council of Medical Research (ICMR) have reported a reduced prevalence of HCC compared to South East Asia, Japan, and European nations [41].
USA
HCC is the most rapidly developing form of cancer in the US, with an increasing incidence and death rate and poor survival [45, 49, 50]. HCV, HBV, alcohol-related liver disease (ALD), and metabolic conditions like non-alcoholic fatty liver disease (NAFLD), obesity, and type-2 diabetes are all significant causes of liver cancer. El-serag et al. [51], Chang et al. [52], Pinheiro et al. [53], and Miller et al. [54] found that these diseases have different profiles depending on the race, gender, and place of birth, which contributes to a considerable variation in a diverse population. It has been noted that chronic HCV infection affects every age group; however, it mainly affects the 1945–1965 birth cohort, which has been a significant driver of increases in incidence and death from liver cancer and HCC in the US [51, 55]. Therefore, the Centers for Disease Control and Prevention (CDC) encourages people to undergo HCV screening for the “baby boomers” cohort in the US since their HCV prevalence is up to five times more than the national average [55].
Compared to other examined demographic categories, including non-Hispanic whites and Asians, Rican and African Americans (US-born), males exhibited a relatively increased age-specific rate (ages 50–69). Surprisingly, this category demonstrated more excellent rates than their senior age equivalents (ages 70–74), providing a confusing “hump and dip” figure rather than the gradual rises in death due to HCC that are generally found as people get older [56]. However, if the typical broad groups of “Hispanic” and “Black” were studied, these significant variations in HCC threat presumably connected with HCV would have been ignored. Death due to liver cancer cases and associated disparities among the older generation are exceptionally substantial in minority populations in previous research [56]. However, in most cases, the surplus is measured concerning Whites, the most influential ethnic group in the US. Therefore, such death rates among the US white senior population can be expected on the other hand. Figure 4 depicts this trend in the number of HCC cases across the US population.
Liver cancer mortality cases across the United States. a, b Age-specific, sex-stratified liver cancer mortality rates by selected racial/ethnic groups: California, Florida, and New York in 2012–2016. c, d Age-specific, sex-stratified liver cancer mortality rates, US Whites and Northern and Western Europeans. US Whites include California, Florida, and New York in 2012–2016; Northern and Western Europeans in 2012–2015. Tan regions highlight areas where the 1945–1965 birth cohort overlaps with younger or older cohorts. Reproduced with permission from [57], copyright@2019 (Elsevier)
Among males and women, the fraction of the overall mortality rates among the people from the 1945–1965 birth cohorts were most significant in the US-born categories. African American men and women showed the most significant relative liver cancer-based mortality rates, 59% for males and 46% for women, during the 1945–1965 cohort. Except for the South Asian females, all communities with the most foreign-born residents had a relatively modest part in the 1945–1965 generation. Among the 1945–1965 cohorts, the lowest death rates were seen among the South Asian males (24%) and Japanese females (19%). As per the above studies, it was concluded that the mortality rates for liver cancer and HCC have increased with an increase in age. Notably, Mexican immigrants, South/Central Americans, and Asian males have increased death rates with age increases. However, rates decline among African American and Puerto Rican males at an unexpectedly low rate as they become older. In addition, except for African Americans, females' age-specific rates increase with age for all ethnic groupings. Finally, US Whites displayed a constant trend based on age and birth year groupings compared to Northern and Western Europeans. Males in the US had a 49% greater death risk due to liver cancers than men in Northern and Western Europe, between 50 and 64%.
Gulf Cooperation Council
Countries including Qatar, Oman, Bahrain, Saudi Arabia, and the United Arab Emirates (UAE) make up the Gulf Cooperation Council (GCC). There are conflicting data on the frequency and mortality rates related to HCC in the GCC. However, most research to date has listed HCV as the most prevalent (approximately 45% cause of HCC, followed by HBV infection at 27%) of the cases [58]. The substantial increase in the number of HCC cases, on the other hand, indicates that the cause of HCC may soon shifts from chronic viral liver disease to non-viral liver disease. HBV and HBC infections have been reported to cause 70% of HCC cases detected in GCC. HCV has been found in high incidence in the general population of nations such as Bahrain (1.2%), the UAE (1.3%), Qatar (1.6%), and Egypt (6.3%) [59].
Between 1990 and 2017, the incidence rate of HCV-related HCC rose by 16.1%. Egypt and Qatar are dominant countries that plan to eliminate HCV by 2030 [60, 61]. From 1990 to 2017, the ASIR for liver cancer grew by 11.91%. Between 1990 and 2017, the ASIR of liver cancer rose in the UAE (+ 27.68%), Kuwait (+ 12.46%), Qatar (− 33.79%), Oman (− 7.61%), Saudi Arabia (+ 0.94%), and Bahrain (− 24.34%). Figure 5 discusses this trend in the number of cases across the GCC.
Trends in HCC cases in the GCC in 1990 and 2017. a The graph represents the incidence and mortality rates of liver cancers in 1990 and 2017. b Graphical representation of underlying liver cancer disease conditions (HBV, HCV, NASH, ALD, others) in 1990 across the GCC. c Graphical representation of underlying liver cancer disease conditions (HBV, HCV, NASH, ALD, others) in 2017 across the GCC. Data were taken with permission from [62], copyright@2020 (Wiley)
Selected Plants and Their Anticancer Activity Against Hepatocellular Carcinoma
Natural herbal medicines have been shown to possess several health benefits [63,64,65,66]. Many natural plants have been shown to possess the ability to treat liver cancers and cirrhosis; these are mentioned in Fig. 6.
Asparagus Racemosus
The genus Asparagus, widespread in India, Asia, Australia, and Africa, includes 300 species globally [67]. Historical sources show the use of plants from the genus over centuries in classical medicine. Asparagus racemosus is a perennial plant, which grows as tall as 7 m (23 ft) and belongs to the family Asparagaceae, which possess a stiff stem, needle-like leaves, and small white flowers. Shatavari (A. racemosus) is a well-known Ayurvedic Rasayana that helps to slow down the aging process and boost immunity, neuropathy, and hepatopathy [68]. It is also used for the preparation of Chyawanprash [69,70,71]. Various studies have revealed that aqueous root extracts of A. racemosus exhibit anticancer activity, anti-inflammatory, antioxidant, and immunomodulatory properties [72].
Extractions are derived from dried roots of A. racemosus. The main pharmacological compounds synthesized by A. racemosus include steroidal saponins, essential oils, various amino acids, flavonoids, resin, steroidal tannin glycosides (asparaguses), and bitter glycosides. Other primary chemical constituents reported from roots and leaves of A. racemosus include diosgenin and shatavarins I and IV [73]. These saponin glycosides derived from the roots of A. racemosus can prevent hepatocellular carcinoma experimentally induced by diethylnitrosamine (DEN). When the hepatic tissues of DEN-treated rats are stained immunohistochemically, clusters of cells with mutated p53 antigen are observed. As per this study by Agrawal and colleagues, Wistar rats were given an aqueous extract of racemosus roots, showed no case of hepatocarcinogenesis. Furthermore, DMBA, the induced mammary tumor, showed a sharp decline in rats exposed to A. racemosus. Rats fed with a 2% A. racemosus diet showed decreased tumor cases and the average number of tumors per tumor-bearing specimens, thus signifying the importance of A. racemosus in the treatment HCC [74].
Solanum Nigrum
Solanum nigrum (black nightshade) comes from the family of Solanaceae and is distributed throughout the world, and is available in various forms [75]. The S. nigrum is native to Eurasian and does not occur naturally in South America [76]. Nevertheless, the plant is frequently used as an essential ingredient to treat pneumonia, stomach ulcers, fever, aching tooth, inflammation, etc. According to recent research, the aqueous extract of S. nigrum (AESN) is an essential ingredient in typical Chinese medicine formulations to treat patients with different cancers [77]. The S. nigrum has antitumor effects against human melanoma, colorectal, endometrial, breast, and liver cancers [78,79,80,81]. In addition, S. nigrum exhibits antiproliferative properties in various cancer cells [82, 83], suppressing tumor cell growth primarily through apoptosis induction. Solanum nigrum is rich in secondary metabolites such as alkaloids and steroid saponins glycoprotein that exhibit anticancer activity [84, 85]. Recent research has discovered four novel steroidal glycosides, alkaloids solamargine, solasonine, alpha, and beta solanigrinechez, inhibit cell proliferation and cause apoptosis in uncontrolled cancers [86]. Water extracts of S. Nigrum protect rats from CCl4-induced chronic hepatotoxicity [87]. By modulating the antioxidative defense mechanism, S. nigrum can minimize CCl4-induced lipid peroxidation. Elshater et al. discovered that rats given S. nigrum after 30 days after the CCl4 challenge showed a substantial decrease in liver marker enzymes, lipid peroxidation, and an increase in enzymatic and non-enzymatic antioxidative defense mechanism [88].
Rubus Aleafolious Poir
Rubus aleaefolius, commonly called Elm-Leaf Blackberry, belongs to a family of Rosaceae and is native to Europe, Africa, and is also naturalized in Kashmir. The R. aleaefolius is a folk medicine used to cure a variety of hepatic diseases, including HCC. The R. aleaefolius contains numerous essential medicinal chemicals, including butanol and ethyl acetate, has shown hepatoprotective properties in mice with acute liver damage following exposure to CCl4, as per the study carried out by Hong et al. Zhao et al. scrutinize extractions of total alkaloids from R. aleaefolius (TARAP), used as an antimetastasis medication to treat HCC in both in vitro and in vivo conditions.
HCC development has shown to be influenced by TARAP with the induction of apoptosis via the caspase 3 and 9 pathways in HepG2 cells and by mitochondrial-mediated apoptosis [89]. Many human cancers, including HCC, have constitutively activated signal transducer and activator of transcription 3 (STAT3) pathway, which plays an essential role in cell proliferation and multiplication in such cancers. This STAT3 phosphorylation can be suppressed in tumor tissues by the TARAP treatment [90]. Furthermore, TARAP has been shown to change the expression of several primary STAT3 signaling pathway target genes, like cyclin D1, cyclin E, cyclin-dependent kinase (CDK) 4, and CDK2 as up-regulating p21. These findings confirm that one of TARAP’s anticancer activity mechanisms against HCC is inhibiting the STAT3 signaling pathway, leading to cell multiplication block and thus arresting the cell cycle.
Perilla Frutescens
The annual herb Perilla frutescens is commonly known as Korean perilla or Beefsteak vine. Perilla’s origins can be traced back to China, Japan, Korea, Taiwan, and Vietnam. This herb is a member of the mint family, Lamiaceae, and can be found across Asia and is assessed in its contribution to culinary and traditional medicinal uses. Extractions from this plant’s seed, leaf, and stem parts are used for many therapeutic applications such as poisoning, bloating, cold, and headaches [91]. In addition, P.a frutescens has been demonstrated to possess anticancer and antitumor efficacy both in vivo and in vitro conditions.
The P. frutescens leaf extract had the best anticancer efficacy in Hep G2 cells, inhibiting cell growth and increasing apoptosis-related gene expression [92]. In addition, Lin et al. used leaf extracts of P. frutescens to evaluate its inhibitory effects in human hepatoma Hep G2 cells. They discovered that it efficiently activates apoptosis-related genes and inhibits cell growth. Other investigations found that an ethanolic leaf extract of P. frutescens increased apoptosis and tumor formation by combining death-receptor-mediated apoptosis with the scavenging of reactive oxygen species (ROS) [93, 94].
Ajwa Dates (Phoenix dactylifera L.)
The Al-Madinah Al Munawara and its surrounding areas in Saudi Arabia are home to the most common natural fruits, mainly date fruit (Phoenix dactylifera L.) [95]. The anticholesteremic, antidiabetic, anti-inflammatory, antioxidant, hepatoprotective, and anticancer properties of the Ajwa date have been identified in conventional and alternative medicines [96, 97]. Phytochemicals in Ajwa fruits (flavonoids glycosides, polyphenol, and phytosterols) [98, 99] exhibit anti-inflammatory, antioxidant, cardioprotective, and antiapoptotic properties [100]. An aqueous extract of Ajwa dates was found to reduce and block diethylnitrosamine-induced liver carcinoma in a rat model [101]. Pulp also includes phenolics, including quercetin and kaempferol [99], which have been shown to have anticancer activity against HCC cells, according to other reports [102, 103].
Anticancer Activity of Extracts and Compounds from Curcuma longa and Its Treatment for Hepatocellular Carcinoma
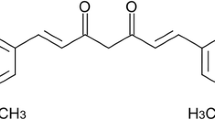
In recent years, there has been a revival of interest in studying the usage and effectiveness of medicinal herbs to treat various diseases. In this section, we have performed a deep analysis of the properties of Curcuma longa for the treatment of liver cancers, particularly HCC, and have also discussed its mechanism of action in detail. Curcumin, with the scientific name “Curcuma longa,” the chemical name “diferuloylmethane,” and the chemical formula C21H20O6, is an essential ingredient of turmeric plants.
Chemical Structure of Curcumin
Curcumin, a yellow pigment from C. longa, makes up 2–8% of turmeric compounds and is generally used as a food coloring agent and spice. The C. longa belongs to the Zingiberaceae plant family and is widely distributed in south-eastern and southern tropical Asia [104]. Rhizomes are the most commonly used plant component. They contain many compounds, including bioactive non-volatile curcuminoids like curcumin, dimethoxy, bisdemethoxycurcumin, and volatile oil compounds like sesquiterpenoids [105, 106]. Due to its hydrophobic nature, curcumin is almost insoluble in a neutral solvent (i.e., water) and readily soluble in organic solvents, and possess low inherent toxicity and properties including antioxidant, anti-inflammatory, antimicrobial, antitumor, antidiabetic, hypocholesterolemic, antithrombotic, antihepatotoxic, antidiarrheal, carminative, diuretic, antirheumatic, hypotensive, antioxidant, larvicidal, insecticidal, antivenomous, and antityrosinase effects [107,108,109,110]. Curcumin’s anticancer activity is recognized as one of its most important effects, owing to its low cytotoxicity to normal cells. Antiproliferative effects have also been observed in various cancer cell lines, including prostate, breast, colorectal, pancreatic, and kidney cancers [111]. When consumed as 0.1–3 mg/kg body weight, curcumin has been shown to inhibit the telomerase reverse transcriptase enzyme and lower Bcl-2 expression [112, 113].
Mechanism of Action of Curcumin
Curcumin has been shown to inhibit carcinogenesis by influencing angiogenesis and cancer cell development. It also indicates the antiangiogenesis effect by inhibiting angiogenic factor stimulators, including VEGF and primary fibroblast growth factor. Curcumin inhibits IL-8 expression by blocking VEGF expression through NF-kB, AP-1 regulation [114], and the PI3K/Akt signaling pathway [115]. Curcumin also suppresses angiogenic cytokines, including IL-6, 23, and 1, which inhibit angiogenesis in certain tumors. It may also cause cancer cells to die by inducing apoptosis via a p53-dependent pathway. Several studies have shown that curcuminoid compounds serve as free-radical scavengers by minimizing lipid peroxidation caused by free radicals [116]. Curcumin-mediated suppression of nuclear factor B (NF-B) allows regulating the inflammatory cascade in most chronic illnesses, including cancers [117]. This can be better understood from Fig. 7 that describes the mechanism of curcumin action in HCC treatment.
Mechanism of curcumin action in the treatment of HCC. a The postulated mechanism for the effect of curcumin on autophagic and apoptotic pathways and ROS in thioacetamide-induced HCC. Adapted with permission from [118], copyright@2017 (Elsevier). b Cellular and molecular mechanisms of curcumin in the prevention of oxidative-associated liver disease. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), ROS, sulfasalazine reduces superoxide dismutase (SOD), glutathione (GSH), glutathione reductase (GR), malondialdehyde (MDA), catalase (CAT), inducible nitric oxide (iNOS), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), extracellular signal-regulated kinases (ERK), and mitogen-activated protein kinase (MAPK). Adapted with permission from [119], copyright@2018 (MDPI). c Biological activities of curcumin. c Mechanism of oxidative bioactivation of curcumin, initiated by hydrogen abstraction from a phenolic hydroxyl of curcumin. The resulting quinone methide radical forms a cyclopentadiene ring and adds molecular oxygen to give a spiroepoxide intermediate that undergoes further transformation to the final dicyclopentadiene. Adapted with permission from [120], copyright@2017 (JBC)
Various studies have shown that curcumin has a high potential for treating various inflammatory diseases [120,121,122] and can (i) block pro-inflammatory transcription factors (NF-kB and AP-1); (ii) decrease pro-inflammatory cytokines TNFα, IL-1b, IL-2, IL-6, IL-8, MIP-1a, MCP-1, CRP, and PGE2; (iii) down-regulate enzymes such as 5-lipoxygenase and COX-2 and COX-5; and (iv) inhibit MAPK and pathways involved in nitric oxide synthase (NOS) enzymes synthesis [121, 123,124,125].
Curcumin Stimulates Apoptosis of Liver Cancer Cells
Curcumin can stimulate apoptosis of liver cancer cells [126]. Extrinsic and intrinsic mechanisms of action of curcumin can both induce programmed cell death. Internal stimuli such as DNA abnormalities, ischemia, viral infection, and cellular distress activate the intrinsic route [126]. Death receptors from the TNF receptor gene superfamily are involved in the extrinsic (receptor-mediated) pathway [127]. According to a study by Liu et al. EF24, a synthetic molecule and a robust curcumin analog with increased bioavailability can effectively decrease HCC and promoted apoptosis in a mouse liver cancer cell line [128]. Compared to control (non-EF24-treated) groups, authors noted that cytochrome c, cleaved-PARP, Bax, and activated caspase-3 levels were high, whereas PARP and Bcl-2 were down. In addition, curcumin treatment of human hepatoma SMMC-7721 cells for 24 h can lower BCL-2 protein expression [129]. Another study on mouse liver cancer cells observed that EF24 causes cell cycle arrest in the G2/M phase. The activation of CDC-2 by cyclin B1 is required for the transition from G2 to M-phase. The authors recorded that the cells’ levels of cyclin B1 and CDC-2 were dramatically lowered when curcumin was used [128]. In addition, curcumin administration prompted the activation of the Chk1-mediated G2 checkpoint, resulting in G2/M arrest and resistance of malignant cells to curcumin-induced apoptosis, as shown in Fig. 8.
Molecular targets of curcumin when used against liver cancers. Reused with permission after changing the design from [130], copyright@2020 (Frontiers)
In another study by Wang and coworkers also revealed the apoptotic pathway of curcumin in treating liver cancers. In addition, the authors demonstrated that the apoptosis-inducing effect of curcumin is linked with mitochondrial apoptosis. Herein, upon the secretion of cytochrome c, caspase-9 gets activated, which cleaves caspase-3 and polymerase 1, which then paves the path to cell death [131].
Curcumin Prevents Metastasis and Tumor Progression
TNF-α has a critical function in tumor cell survival and malignancy. This TNF-α expression can be inhibited by curcumin. However, curcumin's hydrophobicity and limited bioavailability can become substantial roadblocks. To overcome this, curcumin can be encapsulated in microcells to create a sustained release formulation and thus improve its solubility and bioavailability [132]. Compared to the free form of curcumin, such curcumin-bearing microcells can dramatically lower the levels of liver enzymes in the HCC-induced animal groups. In addition, curcumin-containing microcells can also stimulate the production of pro-apoptotic molecules such as p53 and Bax. Figure 9 explains the curcumin action in tumor progression prevention and metastasis.
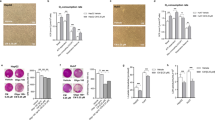
Curcumin action in treating liver cancers. a Illustration of the mechanism of curcumin compound (GA–GFFYK(Cur)E-ss-ERGD) for tumor targeting therapy. The compounds were created by modifying curcumin supramolecular pro-gelator (GA-Cur) with glycyrrhetinic acid (GA) and a compound Nap-Cur after displacing GA with the naphthylacetic acid (Nap). Adapted with permission from [133], copyright@2017 (Nature). b Schematic diagram showing the action of Janus magnetic mesoporous silica nanocarriers for magnetically targeted and hyperthermia-enhanced curcumin therapy of liver cancer. Adapted with permission from ref. [134], copyright@2018 (RSC). c (i and ii) Effects of curcumin on the hepatic GRP78 protein expression and the ratio of p-PERK/PERK and-IRE1α/IRE1α in alcohol-induced liver injury. Adapted with permission from [135], copyright@2019 (Food & Nutrition Research). d (i and ii) Representative flowcharts for a negative detection or a positive detection of circulating tumor cells by flow cytometry showing the reduction of stem cells (s.c.) grafted primary gastric cancer cells (PGCs) in the presence of curcumin. e Curcumin reduces circulating tumor cells (CTCs) of s.c. They grafted PGCs. The ratio of detection of CTCs. *p < 0.05. N = 30. Adapted with permission from [136], copyright@2019 (Aging). f The reversal effects of curcumin on peripheral immunological cells. Ex vivo curcumin treatment of peripheral blood mononuclear cells from patients with NAFLD resulted in decreases in (i) linoleic acid-induced ROS generation and (ii) leptin-induced TNF-α production by monocytes. (iii) Ex vivo curcumin treatment of peripheral blood mononuclear cells from patients with NAFLD resulted in decreased IFN-γ production in CD4 + cells. Lines connect the “linoleic acid” and “linoleic acid + curcumin” stimulation indexes or the “leptin” and “leptin + curcumin” fold of increase indexes for each patient. Adapted with permission from [137], copyright@2017 (PLOS ONE). g Curcumin reduces metastatic tumor formation in the liver of s.c. Grafted pooled primary gastric cancer cells that also affected the hepatic cells. Representative images show a positive and a negative case by bioluminescence in the liver area. Adapted with permission from ref. [136], copyright@2019 (Aging). h Effects of curcumin (CUR) on a high-fat diet (HFD)-induced hepatic steatosis. NC: Normal cells. Adapted with permission from ref. [137], copyright@2021 (Frontiers)
Curcumin’s Effect on the Biochemical Profile
Choudhury et al. found that a curcumin injection (8.98 M) lowered NADH oxidase and elevated GR, GST, and succinate dehydrogenase activity in Swiss albino rats with CCl4-induced hepatotoxicity [138]. Curcumin treatment (200 mg/kg) in Sprague–Dawley rats enhanced hepatic glutathione levels. It lowered lipid peroxidase levels and the activities of both ALT and aspartate AST for the same kind of hepatotoxicity [139]. Curcumin may thus be a potential drug for preventing oxidative stress-related liver disease by lowering ALT, AST, and alkaline phosphate levels, raising GST, GR, GPx, SOD, and CAT levels, and reducing NO and suppressing ROS generation [119]. In addition, curcumin therapy can boost endogenous antioxidant levels (ascorbic acid, GSH, SOD, and CAT) in the liver of chronic iron overloaded male rats, according to Badria et al. [140].
Positive and Negative Side Effects of Using Curcumin to Treat Hepatocellular Carcinoma
With the recent advances in science and technology, several options serve as therapeutic options to treat hepatocarcinoma [141]. Pathogenesis of hepatocarcinoma is a multi-step process, and the extent of angiogenesis determines the therapeutic option to be used. Most common include surgery, radiofrequency ablation, embolization, and liver transplant. Immunotherapeutic prospects are also promising to treat melanomas; the treatment option used to treat HCC depends on the cancer stage. Recently, trans-arterial chemoembolization (TACE) has been combined with sorafenib to block neovascularization during tumour development and proliferation [142]. Though these methods are often successful, conditions like patient’s response and performance and liver function are also crucial, determining factors for HCC treatment.
Further research needs to be done to avoid uncertainties and side effects these treatments follow. For instance, though TACE in combination with sorafenib has shown positive results, it is still uncertain to recommend their exact dosage, frequency, and duration during the entire treatment. In addition, recent studies have deemed the newer modalities ineffective and do not provide any therapeutic benefit during the treatment of HCC [143]. Also, with the increasing medical expenses, it’s difficult for all patients to afford the prescribed option for targeted therapy. High death rates hinder most of the available HCC treatment options due to the recurrence of cancer or resistance of tumours against the therapeutic option used [144]. Options like radiation therapies are minimal in terms of the advanced prognosis of the tumour. Radiotherapy does not allow for precise location of the tumour margins and often destroys the normal cells in the patients. Therefore, alternative treatment options are necessary to increase their response rate and lower the toxicity resulting from the treatment. This is why curcumin serves as a promising therapeutic option for HCC treatment.
The significant advantage of curcumin as a chemotherapeutic agent is its ability to be used alone or in combination with other potential chemotherapeutics without showing any adverse effects like neurotoxicity or peripheral neuropathy [145]. Furthermore, its anti-inflammatory, hepatoprotective, antimutagenic properties, curcumin also helps combat issues like gastrointestinal inflammation that arise due to chemotherapies or radiotherapies [146]. Post chemotherapy, patients are prone to multiple infections. Curcumin, possessing anti-infective properties, easily helps overcome major and minor ailments [147]. Other positive side effects of curcumin include its effect on cell-cycle progression, invasion, epithelial-mesenchymal transition, and drug resistance shown by cancer cells [148]. Furthermore, Curcumin has been shown to perform immunoregulation of HCC by monitoring the miR-21/TIMP3 axis [149]. Shao et al. demonstrated that curcumin suppresses tumour proliferation by down-regulating lincROR and inactivating the Wnt/β-catenin signalling, thus placing a crucial anticancer role during the treatment of HCC [150]. In another study by Bose and colleagues, the authors concluded that curcumin affected the ratio of CD4 + T cells to CD8 + T cells by lowering the regulatory T-cells and reducing the T-cell apoptosis and thus enhancing the immune response against cancer cells in HCC [151].
A recent study by Tian and colleagues showed that when used along with drugs like paclitaxel, curcumin enhances the body’s response towards HCC treatment and reduces chemoresistance [152]. In another similar study, curcumin, piperine, and taurine, when used in combination, affect interleukin-10 and miR-21 levels and, therefore, increase anticancer activity in HCC patients [153]. Likewise, in another study by Man and coworkers, it was concluded that the addition of curcumin in diet strengthened the overall antitumor efficacy in the body and overcame most limitations posed by current treatment options like sorafenib [154]. Thus, curcumin can be combined with other existent options to treat hepatocellular carcinoma better.
In addition, curcumin also suppresses the activity of pro-angiogenic proteins, like vascular endothelial growth factor [23], COX-2, and basic fibroblast growth factors; inhibits cell motility, cellular adhesion molecules, endothelial cell migration, metastasis and related complications, and extracellular proteolysis [155]. Furthermore, curcumin displays antiproliferative, low immunogenicity, and proapoptotic effects on cancer cells [149]. These exceptional properties of curcumin, in addition to no toxic effects, show promising potential for curcumin to be used in combination with other techniques for the treatment of hepatocellular cancer.
Generally, curcumin is considered safe for moderate consumption and is commonly used in South Asian households. However, few cases of colorectal carcinoma have been reported in association with curcumin when consumed in large doses [156]. When ingested in raw form and large quantities, curcumin may lead to gastric irritation, indigestion, diarrhea, allergies, and antithrombosis activity [157]. Apart from its antioxidative properties, curcumin also shows pro-oxidative properties [158]. Based on its concentration, curcumin may either function as an antioxidative agent or as a pro-oxidative agent. Therefore further research must be done to understand better how curcumin functions under changing conditions.
Risk Factors of Hepatocellular Carcinoma
HBV
Hepatitis B virus (HBV) is thought to be the most potent epidemiological factor linked to HCC. Nearly half of all HCC cases are caused by chronic hepatitis B (CHB). However, the significance of risk factors varies significantly by area (e.g., more in East Asia but lower in Europe) [159]. More than 250 million people worldwide are affected with chronic HBV or have been exposed to it. Infants are particularly more at risk. About 90% of children infected with HBV become chronic HBV carriers [160]. The rate of HBV carriers is nearly 8% in high endemic regions [161]. In high-incidence areas, 80% of HCC patients show the Hepatitis B surface antigen (HBsAg) [162]. In untreated CHBV patients, the 5-year average incidence of cirrhosis is 8–20%, with a 2–5% annual chance of HCC in people with cirrhosis. Every year, nearly 900,000 people die from HCC caused by HBV. According to WHO, post-COVID-19 era, there will be an additional 5.3 million chronic HBV infections in children born between 2020 and 2030 and an estimated a million deaths due to HBV among them. In addition, the number of children below five years with chronic HBV infection has reduced from 5% in the pre-vaccine period to just under 1% in 2019 [163]. Socio-demographic factors like gender, age, family history, viral etiology, environmental exposure (aflatoxin intoxication, tobacco, alcohol), and dietary factors are linked to higher HCC affliction chances [164, 165]. HBV-driven HCC can develop either via direct or indirect mechanisms, which includes the following: (1) ongoing inflammatory processes that attempt at clearing the disease condition; (2) changes in host genome structures that incorporate HBV DNA sequences and changes in epigenetics; and (3) accumulation of altered forms of HBV envelope proteins and continuous expression of viral proteins with oncogenic potentials like the regulatory HBX protein.
HCV
Hepatitis C virus (HCV) is a hepatotropic positive single-stranded RNA virus that causes HCC along with other risk factors. HCV-related HCC has the maximum death rates per 100,000 people in US [166, 167]. HCV etiology is found in 30% of Asian HCC patients and 50% Caucasian HCC patients. During 2009–2018 the number and rate of newly reported chronic HCV cases have rapidly increased among young people, with the highest new infections among 20–29 years of age. As per CDC analysis, cirrhosis is most prevalent in patients with HCV-related HCC being approximately 80–90% [168, 169]. In addition, meta-analysis studies showed that the patients with HCV genotype 3 have a 50% increased rate of fibrosis than other genotypes [170]. The risk factors associated with cirrhosis are explained through pictorial representation in Fig. 10.
In cirrhotic cases, HCC occurs more often than in people with mild fibrosis. Mutations cause HCV-HCC in hepatocytes in the context of cirrhosis. HCV proteins are also connected with cell division, metastasis, tumor development, and transformation. When over-expressed, HCV core proteins NS3 and NS5A block tumor suppressor genes TP53, TP73, and RB1 and inhibit negative cell cycle regulators like CDKN1 [171].
NAFLD/ NASH
Nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) is a benign (or recurrent) form of the disease where, histologically, accumulation of fat occurs (steatosis) in > 5% of the hepatocytes [172, 173]. It is anticipated to become the most prominent reason for end-stage liver disease and HCC [2, 3]. Shortly, the occurrence of NAFLD will be simultaneous with an increase in obesity and the event of metabolic syndrome. Such a combination will represent a severe health hazard, causing an increase in liver-related morbidity and mortality, especially in HCC conditions [174,175,176,177]. The NAFLD/NASH-related mortality post-liver cirrhosis has risen significantly in the last ten years. HCC in individuals with NAFLD/NASH is also detected in people devoid of cirrhotic conditions. This causes a delayed diagnosis and a worsened tumor burden.
Steatosis alone does not cause HCC since the rampant inflammatory condition is needed for carcinogenesis [178]. The NASH and HCC are the product of several concurrently acting factors, such as genetic changes, impaired lipid metabolism, and insulin resistance [177, 179, 180]. HCC production is aided by hepatocyte cell death, compensatory division, and increase in levels of TNF, transforming growth factor (TGF), activation of liver sinusoidal endothelial cells, and hepatocyte chromosomal aberrations [172]. Overproduction of ROS is triggered by increased fatty acid oxidation and hepatocyte metabolism [173]. Excess in triglycerides and free fatty acids (FFAs) prevents autophagy by activating the mammalian target of rapamycin (mTOR). DNA damage and oxidation occur when the antioxidant limit of the hepatocytes is crossed, ultimately leading to cell death [181, 182].
Lifestyle Risk Factors
Alcoholic liver disease (ALD) is a severe disease that affects hepatic metabolism and leads to steatosis, fibrosis, cirrhosis, and liver cancer [183, 184]. Alcohol intake as low as 10 g a day raises the risk of HCC, which accounts for 30% of all liver cancer deaths worldwide [185]. Fatty acid oxidation and lipogenesis are two metabolic pathways that are affected by alcohol consumption [186]. Alcoholic steatohepatitis enhances the production of HCC by up-regulating inflammation, cell proliferation, and mir-122 loss. Genetic variations on alcohol metabolizing enzymes resulting in the accumulation of acetaldehyde (carcinogenic) and thus serves as potential inheritable HCC markers. Alcohol-induced oxidative stress leads to the formation of ROS.its buildup has structural and functional effects on DNA, resulting in cell cycle arrest and death. Alcohol disrupts the synthesis of S-adenosyl-L-methionine (SAMe) and methylation status, all of which are related to the growth of HCC. It is worth noting that the magnitude of ALD is related to genetic susceptibility. PNPLA3 and MBOAT7/TMC4 have also been linked to higher chances of cirrhosis in alcoholics [187].
Environmental Carcinogens
HCC carcinogenesis is linked to a variety of environmental chemicals. Aflatoxin is the most well-known among these. Other factors are exposure to vinyl chloride, arsenic, polycyclic hydrocarbons, and radioactive compounds [188]. Aflatoxins are mycotoxins formed by the fungi Aspergillus flavus and Aspergillus parasiticus and are commonly found in infected grain products like corn, peanuts, and legumes [189, 190]. Aflatoxin B1 causes carcinogenesis in animals and humans by forming DNA adducts with hepatic DNA [191]. In areas with high aflatoxin exposure, as much as a 70-fold increase in chances of HCC has been recorded [192].
Conclusion
Liver cancers, particularly HCC, strongly affect human health worldwide. Unfortunately, despite the advancements in cancer diagnostics and therapeutics, we have not reached the potential to overcome such disease conditions without side effects effectively. Thus, advanced alternative therapies are needed that can overcome such shortcomings. The C. longa and current treatments can be used because they have high antioxidant and anti-inflammatory properties that can help limit human HepG2 cells development. Curcumin has been successfully used with other treatment options like leflunomide, perindopril, and similar antiangiogenic agents to treat HCC [192]. In addition, curcumin has shown the power to affect several signaling pathways, suggesting its antitumor potential. Thus, curcumin might be a good option for preventing oxidative stress-related liver disease by lowering ALT, AST, and ALP levels.
Future Prospective
Furthermore, curcumin, a herb, shows insignificant side effects and is safer and inexpensive than other medicines and therapies. Thus, apart from showing synergistic anticancer effects, it also protects from the potential side effects of chemotherapies. However, there are a few shortcomings to this therapy that still needs to be addressed. For starters, curcumin has low bioavailability, instability, hydrophobicities, and the ability to rapidly clear from the body, hindering its practical usage in clinical applications and as anticancer therapy. This disadvantage can be addressed by functionalizing curcumin with nanomaterials, microcells, liposomes, phospholipid complex before being used as therapeutics. Therefore, further study must be done to understand curcumin’s chemical kinetics and dynamic action and analogs, better analyze curcumin’s therapeutic strategy, and determine the appropriate curcumin dose required for effective HCC treatment.
Thus, the recent findings imply that C. longa is a viable natural product source and promising candidate to be used as an adjuvant in treating HCC. This molecule can be developed into a novel anticancer medicine once its complete apoptotic action has been studied clinically.
Data Availability
All data are given in the manuscript.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- NASH:
-
Nonalcoholic steatohepatitis
- HBV:
-
Hepatitis B virus
- NAFLD:
-
Nonalcoholic fatty liver disease
- ALD:
-
Alcoholic-related liver disease
- CHB:
-
Chronic hepatitis B
- CDC:
-
Centers for Disease Control and Prevention
- GCC:
-
Gulf Cooperation Council
- DMBA:
-
Dimethylbenz(a) anthracene
- AESN:
-
Aqueous extracts of Solanum nigrum
- ACE2:
-
Angiotensin-converting enzyme 2
- CCL4:
-
Carbon tetrachloride
- STAT3:
-
Signal transducer and activator of transcription 3
- CDK:
-
Cyclin-dependent kinase
- HepG2:
-
Hepatoma G 2
- ROS:
-
Reactive oxygen species
- BCL2:
-
B-cell lymphoma 2
- VEGF:
-
Vascular endothelial growth factor
- NF-KB:
-
Nuclear factor Kappa light chain enhancer of activated B cells
- AP-1:
-
Activator protein 1
- PI3K:
-
Phosphoinositide 3-kinase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- SOD:
-
Superoxide dismutase
- GSH:
-
Glutathione
- GR:
-
Glutathione reductase
- MDA:
-
Molanodialdehyde
- CAT:
-
Catalase
- iNOS:
-
Inducible nitric oxide
- HDL:
-
High-density lipo-protein
- LDL:
-
Low density lipo-protein
- TG:
-
Triglyceride
- ERK:
-
Extracellular signal-regulated kinases
- MAPK:
-
Mitogen-activated protein kinase
- PARP:
-
Polyadenosine diphosphate ribose polymerase
- TNFα:
-
Tumor necrosis factor-alpha
- MCP-1:
-
Monocyte chemoattractant protein-1
- CRP:
-
C-reactive protein
- PGE2:
-
Prostaglandin E2
- COX:
-
Cyclo-oxygenase
- NOS:
-
Nitric-oxide synthase
- PERK:
-
Protein Kinase-like endoplasmic reticulum kinase
- p-PERK:
-
Phosphor-thr982
- IRE1 α:
-
Inositol-requiring enzyme 1 alpha
- PGCs:
-
Primordial germ cells
- CTCs:
-
Circulating tumor cells
- NADH :
-
Nicotinamide adenine dinucleotide
- NO:
-
Nitric oxide
- TACE:
-
Trans-arterial chemoembolization
- HbsAg:
-
Hepatitis B virus surface antigen
- HBX:
-
Hepatitis B virus X-protein
- NS3:
-
Nonstructural protein 3
- NS5A:
-
Nonstructural protein 5A
- RBI:
-
Retinoblastoma protein
- TGF:
-
Transforming growth factor
- mTOR:
-
Mammalian target of rapamycin
- mir-122:
-
MicroRNA-122
- PNPLA3:
-
Patatin-like phospholipase domain-containing protein-3
- TMC4:
-
Transmembrane channel-like protein 4
- AfB1:
-
Aflatoxin B1
- MBOAT7:
-
Membrane-bound o-acyltransferase domain-containing protein7
References
Erratum: Global cancer statistics. 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer J Clin. 2020. https://doi.org/10.3322/caac.21609.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019. https://doi.org/10.1038/s41575-019-0186-y.
Pérez LM, López SA, Fajes JLH, Martín LC. Hepatocellular carcinoma. Med. 2020. https://doi.org/10.1016/j.med.2020.06.019.
Cheng KC, Lin WY, Liu CS, Lin CC, Lai HC, Lai SW. Association of different types of liver disease with demographic and clinical factors. Biomed. 2016. https://doi.org/10.7603/s40681-016-0016-2.
American Cancer Society. Facts & Figures 2021. American Cancer Society. Atlanta, Ga. 2021 cancer.org | 1.800.227.2345.
Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015. https://doi.org/10.1053/j.gastro.2015.07.056.
Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, El Bcheraoui C, Moradi-Lakeh M, Kyu HH, Barber RM, Wagner J, Cercy K, Kravitz H. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2016. https://doi.org/10.1016/S0140-6736(16)00648-6
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020. https://doi.org/10.3322/caac.21590.
Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, Richardson P, Elserag HB. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011. https://doi.org/10.1053/j.gastro.2010.12.032.
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009. https://doi.org/10.1200/JCO.2008.20.7753.
Howlander N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR. SEER Cancer Statistics Review. J Natl Cancer Inst. 2019.
Frager SZ, Schwartz JM. Hepatocellular carcinoma: Epidemiology, screening, and assessment of hepatic reserve. Curr Oncol. 2020. https://doi.org/10.3747/co.27.7181.
Forner A, Reig ME, Rodriguez De Lope C, Bruix J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010. https://doi.org/10.1055/s-0030-1247133.
Peters GJ, Honeywell RJ. Drug transport and metabolism of novel anticancer drugs. Expert Opin Drug Metab Toxicol. 2015. https://doi.org/10.1517/17425255.2015.1041255.
Kulik LM, Chokechanachaisakul A. Evaluation and management of hepatocellular carcinoma. Clin Liver Dis. 2015. https://doi.org/10.1016/j.cld.2014.09.002.
Zamor PJ, Delemos AS, Russo MW. Viral hepatitis and hepatocellular carcinoma: etiology and management. J Gastrointest Oncol. 2017. https://doi.org/10.21037/jgo.2017.03.14.
Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J Hepatol. 2017. https://doi.org/10.4254/wjh.v9.i21.907.
Iqbal J, Abbasi BA, Ahmad R, Mahmood T, Kanwal S, Ali B, Khalil AT, Shah SA, Alam MM, Badshah H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed Pharmacother. 2018. https://doi.org/10.1016/j.biopha.2018.09.096.
Agarwal G, Carcache PJB, Addo EM, Kinghorn AD. Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol Adv. 2020. https://doi.org/10.1016/j.biotechadv.2019.01.004.
Iqbal J, Abbasi BA, Batool R, Mahmood T, Ali B, Khalil AT, Kanwal S, Shah SA, Ahmad R. Potential phytocompounds for developing breast cancer therapeutics: nature’s healing touch. Eur J Pharmacol. 2018. https://doi.org/10.1016/j.ejphar.2018.03.007.
Avtanski D, Poretsky L. Phyto-polyphenols as potential inhibitors of breast cancer metastasis. Mol Med. 2018. https://doi.org/10.1186/s10020-018-0032-7.
Shehzad A, Qureshi M, Anwar MN, Lee YS. Multifunctional curcumin mediate multitherapeutic effects. J Food Sci. 2017. https://doi.org/10.1111/1750-3841.13793.
Pan Z, Zhuang J, Ji C, Cai Z, Liao W, Huang Z. Curcumin inhibits hepatocellular carcinoma growth by targeting VEGF expression. Oncol Lett. 2018. https://doi.org/10.3892/ol.2018.7988.
Xu MX, Zhao L, Deng C, Yang LU, Wang Y, Guo T, Li L, Lin J, Zhang L. Curcumin suppresses proliferation and induces apoptosis of human hepatocellular carcinoma cells via the wnt signaling pathway. Int J Oncol. 2013. https://doi.org/10.3892/ijo.2013.2107.
Teng CF, Yu CH, Chang HY, Hsieh WC, Wu TH, Lin JH, Wu HC, Jeng LB, Su IJ. Chemopreventive effect of phytosomal curcumin on hepatitis B virus-related hepatocellular carcinoma in a transgenic mouse model. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-46891-5.
Matias D, Rijo P, Pinto Reis C. Phytosomes as biocompatible carriers of natural drugs. Curr Med Chem. 2017. https://doi.org/10.2174/0929867323666161028160855.
Mirzaei H, Shakeri A, Rashidi B, Jalili A, Banikazemi Z, Sahebkar A. Phytosomal curcumin: a review of pharmacokinetic, experimental and clinical studies. Biomed Pharmacother. 2017. https://doi.org/10.1016/j.biopha.2016.11.098.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today, Accessed [08 May 2021].
Mohammadian M, Soroush A, Mohammadian-Hafshejani A, Towhidi F, Hadadian F, Salehiniya H. The incidence and mortality of liver cancer and its relationship with development in Asia. Asian Pacific J Cancer Prev. 2016. https://doi.org/10.7314/APJCP.2016.17.4.2041.
Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Heal. 2016. https://doi.org/10.1016/S2214-109X(16)30143-7.
Armstrong BK. Cancer Epidemiology and Prevention. Int J Epidemiol. 2018. https://doi.org/10.1093/ije/dyy166.
Chimed T, Sandagdorj T, Znaor A, et al. Cancer incidence and cancer control in Mongolia: results from the National Cancer Registry 2008–12. Int J Cancer. 2017;140:302–9. Wiley Online Library CAS PubMed Web of Science®Google Scholar.
Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159: 335- 349.e15.Crossref PubMed Web of Science®Google Scholar.
Petrick JL, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978–2012. Int J Cancer. 2020;147: 317- 330. Wiley Online Library CAS PubMed Web of Science®Google Scholar.
Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group [see comments]. N Engl J Med. 1997;336:1855–9. Crossref CAS PubMed Web of Science®Google Scholar.
Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, Dusheiko G, Feld JJ, Gore C, Griswold MG, Hamid S. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019. https://doi.org/10.1016/S2468-1253(18)30270-X.
Chang M-H, Chen C-J, Lai M-S, Hsu H-M, Wu T-C, Kong M-S, Liang D-C, Shau W-Y, Chen D-S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med. 1997. https://doi.org/10.1056/nejm199706263362602.
Goodarzi E, Ghorat F, Jarrahi AM, Adineh HA, Sohrabivafa M, Khazaei Z. Global incidence and mortality of liver cancers and its relationship with the human development index (HDI): an ecology study in 2018. World Cancer Res. J. 2019.
Brubaker SD, Ward JW, Hiebert L, Morgan RL. Developing an evidence base for the delivery of hepatitis B virus birth dose vaccination: an evidence map and critical appraisal of systematic reviews and guidelines. Clin Liver Dis. 2021. https://doi.org/10.1002/cld.1103.
Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016. https://doi.org/10.1016/j.cmi.2016.07.035.
Dikshit R, Gupta PC, Ramasundarahettige C, Gajalaksmi V, Aleksandrowicz L, Badwe R, et al. Cancer mortality in India: a nationally representative survey. Lancet 2012;379:1807–16.
Acharya SK. Epidemiology of hepatocellular carcinoma in India. J Clin Exp Hepatol. 2014. https://doi.org/10.1016/j.jceh.2014.05.013.
Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, Kumar R, Roy S, Suraweera W, Bray F, Mallath M, Singh PK, Sinha DN, Shet AS, Gelband H, Jha P. Cancer mortality in India: a nationally representative survey. Lancet. 2012. https://doi.org/10.1016/S0140-6736(12)60358-4.
Paul SB, Sreenivas V, Gulati MS, Madan K, Gupta AK, Mukhopadhyay S, Panda SK, Acharya SK. Acharya, Incidence of hepatocellular carcinoma among Indian patients with cirrhosis of liver: An experience from a tertiary care center in northern India. Indian J Gastroenterol. 2007.
Hunt R, Armstrong D, Katelaris P, Afihene M, Bane A, Bhatia S, Chen MH, Choi MG, Melo AC, Fock KM, Ford A, Hongo M, Khan A, Lazebnik L, Lindberg G, Lizarzabal M, Myint T, Moraes-Filho JP, Salis G, Lin JT, Vaidya R, Abdo A, Lemair A, Melberg J. World Gastroenterology Organisation Global Guidelines. J Clin Gastroenterol. 2017. https://doi.org/10.1097/MCG.0000000000000854.
Batham A, Narula D, Toteja T, Sreenivas V, Puliyel JM. Puliyel, Systematic review and meta-analysis of prevalence of hepatitis B in India. Indian Pediatr. 2007.
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004. https://doi.org/10.1053/j.gastro.2004.09.014.
Rogers G, Hewson P, Wright D, Anderson R, Cramp M, Jackson S, Ryder S, Price A, Stein K. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess. 2007. https://doi.org/10.3310/hta11340.
Rinaldi L, Nevola R, Franci G, Perrella A, Corvino G, Marrone A, Berretta M, Morone MV, Galdiero M, Giordano M, Adinolfi LE, Sasso FC. Risk of hepatocellular carcinoma after hcv clearance by direct-acting antivirals treatment predictive factors and role of epigenetics. Cancers (Basel). 2020. https://doi.org/10.3390/cancers12061351.
Chu CM. Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2000. https://doi.org/10.1046/j.1440-1746.2000.02097.x.
Tanaka K, Sakai H, Hashizume M, Hirohata T. A long-term follow-up study on risk factors for hepatocellular carcinoma among Japanese patients with liver cirrhosis. Japanese J Cancer Res. 1998. https://doi.org/10.1111/j.1349-7006.1998.tb00520.x.
Paul SB, Sreenivas V, Gulati MS. Incidence of hepatocellular carcinoma among Indian patients with cirrhosis of liver: an experience from tertiary care center in northern India. Indian J Gastroenterol. 2007;26:274–278. [PubMed] [Google Scholar].
Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, Anderson RN, Ma J, Ly KN, Cronin KA, Penberthy L, Kohler BA. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016. https://doi.org/10.1002/cncr.29936.
Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, Thomas CC, Islami F, Weir HK, Lewis DR, Sherman RL, Wu M, Benard VB, Richardson LC, Jemal A, Cronin K, Kohler BA. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 2020. https://doi.org/10.1002/cncr.32802.
El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007. https://doi.org/10.1001/archinte.167.18.1983.
Chang ET, Yang J, Alfaro-Velcamp T, So SKS, Glaser SL, Gomez SL. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol Biomarkers Prev. 2010. https://doi.org/10.1158/1055-9965.EPI-10-0863.
Pinheiro PS, Callahan KE, Siegel RL, Jin H, Morris CR, Trapido EJ, Gomez SL. Cancer mortality in hispanic ethnic groups. Cancer Epidemiol Biomarkers Prev. 2017. https://doi.org/10.1158/1055-9965.EPI-16-0684.
Miller KD, Goding SA, Ortiz AP. Fedewa SA, Pinheiro PS, Tortolero-Luna G, Martinez-Tyson D, Jemal A, Siegel RL. Cancer Statistics for Hispanics/Latinos, 2018. CA. Cancer J Clin. 2018. https://doi.org/10.3322/caac.21494.
Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel, Alter M, Yartel A, Ward JW. Centers for Disease Control, Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012.
Pinheiro PS, Callahan KE, Boscoe FP, Balise RR, Cobb TR, Lee DJ, Kobetz E. Cancer site–specific disparities in New York, including the 1945–1965 birth cohort’s impact on liver cancer patterns. Cancer Epidemiol Biomarkers Prev. 2018. https://doi.org/10.1158/1055-9965.EPI-18-0194.
Pinheiro PS, Callahan KE, Jones PD, Morris C, Ransdell JM, Kwon D, Brown CP, Kobetz EN. Liver cancer: A leading cause of cancer death in the United States and the role of the 1945–1965 birth cohort by ethnicity. JHEP Reports. 2019. https://doi.org/10.1016/j.jhepr.2019.05.008.
Rasul KI, Al-Azawi SH, Chandra P, Abou-Alfa GK, Knuth A. Status of hepatocellular carcinoma in Gulf region. Chinese Clin Oncol. 2013. https://doi.org/10.3978/j.issn.2304-3865.2013.11.02.
Blach S, Zeuzem S, Manns M, Altraif I, Duberg AS, Muljono DH, Waked I, Alavian SM, Lee MH, Negro F, Abaalkhail F. Razavi, Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study, Lancet Gastroenterol. Hepatol. 2017. https://doi.org/10.1016/S2468-1253(16)30181-9.
Omran D, Alboraie M, Zayed RA, Wifi MN, Naguib M, Eltabbakh M, Abdellah M, Sherief AF, Maklad S, Eldemellawy HH, Saad OK, Khamiss DM, El Kassas M. Towards hepatitis C virus elimination: Egyptian experience, achievements and limitations. World J Gastroenterol. 2018. https://doi.org/10.3748/wjg.v24.i38.4330.
Derbala M, AbdFarag E, Al-Romaihi H, Al Kaabi S, Al-Thani M, El Sayed E, Amer A, Himatt S. Himatt, An overview of the hepatitis c control plan in qatar. East Mediterr Heal J. 2019. https://doi.org/10.26719/emhj.18.046.
Sharafi H, Alavian SM. The Rising Threat of Hepatocellular Carcinoma in the Middle East and North Africa Region: Results From Global Burden of Disease Study 2017. Clin Liver Dis. 2019. https://doi.org/10.1002/cld.890.
Wood DM, Athwal S, Panahloo A. The advantages and disadvantages of a “herbal” medicine in a patient with a diabetes mellitus: A case report. Diabet Med. 2004. https://doi.org/10.1111/j.1464-5491.2004.01202.x.
Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic applications of herbal medicines for cancer patients. Evidence-Based Complement Altern Med. 2013. https://doi.org/10.1155/2013/302426.
Nguyen NH, Nguyen TT, Ma PC, Ta QTH, Duong TH, Vo VG. Potential antimicrobial and anticancer activities of an ethanol extract from bouea macrophylla. Molecules. 2020. https://doi.org/10.3390/molecules25081996.
Duong TH, Beniddir MA, Trung NT, Phan CTD, Vo VG, Nguyen VK, Le QL, Nguyen HD, Le Pogam P. Atypical lindenane-type sesquiterpenes from Lindera myrrha. Molecules. 2020. https://doi.org/10.3390/molecules25081830.
Lock JM, Gaur RD. Flora of the District Garhwal North West Himalaya (With Ethnobotanical Notes). Kew Bull. 2001. https://doi.org/10.2307/4119449.
Vishwakarma R, Goswami P. A review through Charaka Uttara-Tantra, AYU (An Int. Q. J. Res. Ayurveda). 2013. https://doi.org/10.4103/0974-8520.115438.
Ali M. Rasayana therapy in classical literature of Ayurveda: a review. Bull Indian Inst Hist Med Hyderabad. 1998.
Bopana N, Saxena S. Asparagus racemosus-Ethnopharmacological evaluation and conservation needs. J Ethnopharmacol. 2007. https://doi.org/10.1016/j.jep.2007.01.001.
Gautam M, Saha S, Bani S, Kaul A, Mishra S, Patil D, Satti NK, Suri KA, Gairola S, Suresh K, Jadhav S, Qazi GN, Patwardhan B. Immunomodulatory activity of Asparagus racemosus on systemic Th1/Th2 immunity: Implications for immunoadjuvant potential. J Ethnopharmacol. 2009. https://doi.org/10.1016/j.jep.2008.10.028.
Kamat JP, Boloor KK, Devasagayam TPA, Venkatachalam SR. Antioxidant properties of Asparagus racemosus against damage induced by γ-radiation in rat liver mitochondria. J Ethnopharmacol. 2000. https://doi.org/10.1016/S0378-8741(00)00176-8.
Kumar V, Dev S. Chemistry of Ayurvedic Crude Drugs - VII1 1 Part VI, Indian J. Chem., in press. guggulu (Resin From Commiphora Mukul) -6. Absolute stereochemistry of guggultetrols. Tetrahedron. 1987. https://doi.org/10.1016/S0040-4020(01)87799-1.
Agrawal A, Sharma M, Rai SK, Singh B, Tiwari M, Chandra R. The effect of the aqueous extract of the roots of Asparagus racemosus on hepatocarcinogenesis initiated by Diethylnitrosamine. Phyther Res. 2008. https://doi.org/10.1002/ptr.2391.
Särkinen T, Poczai P, Barboza GE, van der Weerden GM, Baden M, Knapp S. A revision of the Old World Black Nightshades (Morelloid clade of Solanum L., Solanaceae). PhytoKeys. 2018. https://doi.org/10.3897/phytokeys.106.21991.
Jagatheeswari D, Bharathi T, Ali HS. Black night shade (Solanum nigrum L.)-an updated overview. Int J Pharm Biol Arch. 2013.
Lai YJ, Tai CJ, Wang CW, Choong CY, Lee BH, Shi YC, Tai CJ. Anti-cancer activity of Solanum nigrum (AESN) through suppression of mitochondrial function and epithelial-mesenchymal transition (EMT) in breast cancer cells. Molecules. 2016. https://doi.org/10.3390/molecules21050553.
Lin HM, Tseng HC, Wang CJ, Chyau CC, Liao KK, Peng PL, Chou FP. Induction of autophagy and apoptosis by the extract of Solanum nigrum Linn in HepG2 cells. J Agric Food Chem. 2007. https://doi.org/10.1021/jf062406m.
Li J, Li Q, Feng T, Li K. Aqueous extract of Solanum nigrum inhibit growth of cervical carcinoma (U14) via modulating immune response of tumor bearing mice and inducing apoptosis of tumor cells. Fitoterapia. 2008. https://doi.org/10.1016/j.fitote.2008.06.010.
Wang HC, Wu DH, Chang YC, Li YJ, Wang CJ. Solanum nigrum Linn water extract inhibits metastasis in mouse melanoma cells in vitro and in vivo. J Agric Food Chem. 2010. https://doi.org/10.1021/jf1022065.
Huang HC, Syu KY, Lin JK. Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells. J Agric Food Chem. 2010. https://doi.org/10.1021/jf101003v.
Wang CK, Lin YF, Tai CJ, Wang CW, Chang YJ, Choong CY, Lin CS, Tai CJ, Chang CC. Integrated treatment of aqueous extract of Solanum nigrum-potentiated cisplatin- and doxorubicin-induced cytotoxicity in human hepatocellular carcinoma cells, evidence-based complement. Altern Med. 2015. https://doi.org/10.1155/2015/675270.
Tai CJ, Wang CW, Chen CL, Wang CK, Chang YJ, Jian JY, Lin CS, Tai CJ. Cisplatin-, doxorubicin-, and docetaxel-induced cell death promoted by the aqueous extract of Solanum nigrum in human ovarian carcinoma cells. Integr Cancer Ther. 2015. https://doi.org/10.1177/1534735415588826.
Tai CJ, Wang CK, Chang YJ, Lin CS, Tai CJ. Aqueous extract of Solanum nigrum leaf activates autophagic cell death and enhances docetaxel-induced cytotoxicity in human endometrial carcinoma cells, evidence-based complement. Altern Med. 2012. https://doi.org/10.1155/2012/859185.
Ram Mohan M, Baba Shankar Rao G, Narender B, Ananda Kumar C, Venkateswara Rao P, Bakshi V. Indian medicinal plants used as immunomodulatory agents: a review. Int J Green Pharm. 2019.
Ahmad S, Zahiruddin S, Parveen B, Basist P, Parveen A, Parveen R, Ahmad M. Indian Medicinal Plants and Formulations and Their Potential Against COVID-19–Preclinical and Clinical Research. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2020.578970.
Ravi V, Saleem TS, Maiti PP, Ramamurthy J. Phytochemical and pharmacological evaluation of Solanum nigrum Linn. African J Pharm Pharmacol. 2009.
Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. Hepatoprotective effects of Solanum nigrum Linn extract against CCl4-iduced oxidative damage in rats. Chem Biol Interact. 2008. https://doi.org/10.1016/j.cbi.2007.08.008.
Salman M, Elshater AE, Mohamed S. The hepato-ameliorating effect of Solanum nigrum against CCl4 induced liver toxicity in Albino rats, Egypt. Acad J Biol Sci C Physiol Mol Biol. 2013. https://doi.org/10.21608/eajbsc.2013.16111.
Zhao J, Chen X, Lin W, Wu G, Zhuang Q, Zhong X, Hong Z, Peng J. Total alkaloids of Rubus aleaefolius Poir inhibit hepatocellular carcinoma growth in vivo and in vitro via activation of mitochondrial-dependent apoptosis. Int J Oncol. 2013. https://doi.org/10.3892/ijo.2013.1779.
Moran DM, Mattocks MA, Cahill PA, Koniaris LG, McKillop IH. Interleukin-6 mediates G(0)/G(1) growth arrest in hepatocellular carcinoma through a STAT 3-dependent pathway. J Surg Res. 2008;147:23–33. https://doi.org/10.1016/j.jss.2007.04.022.
Huang XX, Gao WY, Man SL, Zhao ZY. Advances in studies on saponins in plants of Paris L. and their biosynthetic approach. Chinese Tradit Herb Drugs. 2009.
Lin CS, Kuo CL, Wang JP, Cheng JS, Huang ZW, Chen CF. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J Ethnopharmacol. 2007. https://doi.org/10.1016/j.jep.2007.05.008.
Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004. https://doi.org/10.1093/carcin/bgh034.
Kwak CS, Yeo EJ, Moon SC, Kim YW, Ahn HJ, Park SC. Perilla leaf, perilla frutescens, induces apoptosis and g1 phase arrest in human leukemia hl-60 cells through the combinations of death receptor-mediated, mitochondrial, and endoplasmic reticulum stress-induced pathways. J Med Food. 2009. https://doi.org/10.1089/jmf.2008.1103.
Ragab AR, Elkablawy MA, Sheik BY, Baraka HN. Antioxidant and tissue-protective studieson Ajwa extract: dates from al-Madinah al-Monwarah, Saudia Arabia. J Environ Anal Toxicol. 2013;3:163. [Google Scholar].
Mallhi TH, Qadir MI, Ali M, Ahmad B, Khan YH. Atta-Ur-Rehman, Ajwa date (Phoenix dactylifera): An emerging plant in pharmacological research. Pak. J. Pharm. Sci. 2014.
Hasan M, Mohieldein A. In vivo evaluation of anti diabetic, hypolipidemic, antioxidative activities of saudi date seed extract on streptozotocin induced diabetic rats. J Clin Diagnostic Res. 2016. https://doi.org/10.7860/JCDR/2016/16879.7419.
Al-Farsi MA, Lee CY. Nutritional and functional properties of dates: A review. Crit Rev Food Sci Nutr. 2008. https://doi.org/10.1080/10408390701724264.
Abdul-Hamid NA, Mediani A, Maulidiani M, Shadid K, Ismail IS, Abas F, Lajis NH. Metabolite characterization of different palm date varieties and the correlation with their NO inhibitory activity, texture and sweetness. J Food Sci Technol. 2018. https://doi.org/10.1007/s13197-018-3073-6.
Taleb H, Maddocks E, Morris RK, Kanekanian AK. Chemical characterisation and the anti-inflammatory, anti-angiogenic and antibacterial properties of date fruit (Phoenix dactylifera L.). J Ethnopharmacol. 2016. https://doi.org/10.1016/j.jep.2016.10.032.
Khan MA, Ahmad R, Srivastava AN. Effect of ethyl acetate aroma on viability of human breast cancer and normal kidney epithelial cells in vitro. Integr Med Res. 2017. https://doi.org/10.1016/j.imr.2016.11.004.
Zhou J, Fang L, Liao J, Li L, Yao W, Xiong Z, Zhou X. Investigation of the anti-cancer effect of quercetin on HepG2 cells in vivo. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0172838.
Guo H, Lin W, Zhang X, Zhang X, Hu Z, Li L, Duan Z, Zhang J, Ren F. Kaempferol induces hepatocellular carcinoma cell death via endoplasmic reticulum stress-CHOP-autophagy signaling pathway. Oncotarget. 2017. https://doi.org/10.18632/oncotarget.19200.
Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa MC. Curcumin and health. Molecules. 2016. https://doi.org/10.3390/molecules21030264.
Itokawa H, Shi Q, Akiyama T, Morris-Natschke SL, Lee KH. Recent advances in the investigation of curcuminoids. Chin Med. 2008. https://doi.org/10.1186/1749-8546-3-11.
Lobo R, Prabhu KS, Shirwaikar A, Shirwaikar A. Curcuma zedoaria Rosc. (white turmeric): a review of its chemical, pharmacological and ethnomedicinal properties. J. Pharm. Pharmacol. 2010. https://doi.org/10.1211/jpp.61.01.0003.
Wilson B, Abraham G, Manju VS, Mathew M, Vimala B, Sundaresan S, Nambisan B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J Ethnopharmacol. 2005. https://doi.org/10.1016/j.jep.2005.02.004.
Reanmongkol W, Subhadhirasakul S, Khaisombat N, Fuengnawakit P, Jantasila S, Khamjun A. Investigation the antinociceptive, antipyretic and anti-inflammatory activities of Curcuma aeruginosa Roxb. extracts in experimental animals. Songklanakarin J Sci Technol. 2006.
Lin CM, Sheu SR, Hsu SC, Tsai YH. Determination of bactericidal efficacy of essential oil extracted from orange peel on the food contact surfaces. Food Control. 2010. https://doi.org/10.1016/j.foodcont.2010.06.008.
Angel GR, Menon N, Vimala B, Nambisan B. Essential oil composition of eight starchy Curcuma species. Ind Crops Prod. 2014. https://doi.org/10.1016/j.indcrop.2014.06.028.
Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: From kitchen to clinic. Biochem Pharmacol. 2008. https://doi.org/10.1016/j.bcp.2007.08.016.
Byun SY, Kim DB, Kim E. Curcumin ameliorates the tumor-enhancing effects of a high-protein diet in an azoxymethane-induced mouse model of colon carcinogenesis. Nutr Res. 2015. https://doi.org/10.1016/j.nutres.2015.05.016.
Sakib Hossain D, Bhattacharyya S, Das T, Sa G. Curcumin: the multi-targeted therapy for cancer regression. Front Biosci - Sch. 2012. https://doi.org/10.2741/s272.
Yance DR, Sagar SM. Targeting angiogenesis with integrative cancer therapies. Integr Cancer Ther. 2006. https://doi.org/10.1177/1534735405285562.
Astinfeshan M, Rasmi Y, Kheradmand F, Karimipour M, Rahbarghazi R, Aramwit P, Nasirzadeh M, Daeihassani B, Shirpoor A, Golineghad Z, Saboory E. Curcumin inhibits angiogenesis in endothelial cells using downregulation of the PI3K/Akt signaling pathway. Food Biosci. 2019. https://doi.org/10.1016/j.fbio.2019.04.005.
Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008. https://doi.org/10.1016/j.cbi.2008.05.003.
Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007. https://doi.org/10.1111/j.1753-4887.2007.tb00352.x.
Elmansi AM, El-Karef AA, El-Shishtawy MM, Eissa LA. Hepatoprotective effect of curcumin on hepatocellular carcinoma through autophagic and apoptic pathways. Ann Hepatol. 2017. https://doi.org/10.5604/01.3001.0010.0307.
Farzaei MH, Zobeiri M, Parvizi F, El-Senduny FF, Marmouzi I, Coy-Barrera E, Naseri R, Nabavi SM, Rahimi R, Abdollahi M. Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients. 2018. https://doi.org/10.3390/nu10070855.
Edwards RL, Luis PB, Varuzza PV, Joseph AI, Presley SH, Chaturvedi R, Schneider C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J Biol Chem. 2017. https://doi.org/10.1074/jbc.RA117.000123.
Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009. https://doi.org/10.1016/j.tips.2008.11.002.
Cianciulli A, Calvello R, Porro C, Trotta T, Salvatore R, Panaro MA. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int Immunopharmacol. 2016. https://doi.org/10.1016/j.intimp.2016.05.007.
Panahi Y, Saadat A, Beiraghdar F, Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phyther Res. 2014. https://doi.org/10.1002/ptr.5149.
Urdzikova LM, Karova K, Ruzicka J, Kloudova A, Shannon C, Dubisova J, Murali R, Kubinova S, Sykova E, Jhanwar-Uniyal M, Jendelova P. The anti-inflammatory compound curcumin enhances locomotor and sensory recovery after spinal cord injury in rats by immunomodulation. Int J Mol Sci. 2015. https://doi.org/10.3390/ijms17010049.
He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules. 2015. https://doi.org/10.3390/molecules20059183.
Ziegler DS, Kung AL. Therapeutic targeting of apoptosis pathways in cancer. Curr OpinOncol 2008;20:97–103. https://doi.org/10.1097/CCO.0b013e3282f310f6. PubMed. http://www.ncbi.nlm.nih.gov/pubmed/18043263.
Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001. https://doi.org/10.1016/S0092-8674(01)00237-9.
Liu H, Liang Y, Wang L, Tian L, Song R, Han T, Pan S, Liu L. In vivo and in vitro suppression of hepatocellular carcinoma by EF24, a curcumin analog. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0048075.
Yu J, Zhou X, He X, Dai M, Zhang Q. Curcumin induces apoptosis involving Bax/Bcl-2 in human hepatoma SMMC-7721 cells. Asian Pacific J Cancer Prev. 2011.
Sharifi-Rad J, El Rayess Y, Rizk AA, Sadaka C, Zgheib R, Zam W, Sestito S, Rapposelli S, Neffe-Skocińska K, Zielińska D, Salehi B, Setzer WN, Dosoky NS, Taheri Y, El Beyrouthy M, Martorell M, Ostrander EA, Suleria HAR, Cho WC, Maroyi A, Martins N. Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. 2020. https://doi.org/10.3389/fphar.2020.01021.
Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012. https://doi.org/10.1007/978-94-007-2869-1_7.
Farazuddin M, Dua B, Zia Q, Khan AA, Joshi B, Owais M. Chemotherapeutic potential of curcumin-bearing microcells against hepatocellular carcinoma in model animals. Int J Nanomedicine. 2014. https://doi.org/10.2147/IJN.S34668.
Chen G, Li J, Cai Y, Zhan J, Gao J, Song M, Shi Y, Yang Z. A glycyrrhetinic acid-modified curcumin supramolecular hydrogel for liver tumor targeting therapy. Sci Rep. 2017. https://doi.org/10.1038/srep44210.
Xing H, Wang Z, Shao D, Chang Z, Ge M, Li L, Wu M, Yan Z, Dong W. Janus nanocarriers for magnetically targeted and hyperthermia-enhanced curcumin therapy of liver cancer. RSC Adv. 2018. https://doi.org/10.1039/c8ra05694c.
Wang B, Gao X, Liu B, Li Y, Bai M, Zhang Z, Xu E, Xiong Z, Hu Y. Protective effects of curcumin against chronic alcohol-induced liver injury in mice through modulating mitochondrial dysfunction and inhibiting endoplasmic reticulum stress. Food Nutr Res. 2019. https://doi.org/10.29219/fnr.v63.3567.
Gu X, Zhang Q, Zhang W, Zhu L. Curcumin inhibits liver metastasis of gastric cancer through reducing circulating tumor cells. Aging (Albany. NY). 2019. https://doi.org/10.18632/aging.101848.
Inzaugarat ME, De Matteo E, Baz P, Lucero D, García CC, Ballerga EG, Daruich J, Sorda JA, Wald MR, Cherñavsky AC. New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS ONE. 2017. https://doi.org/10.1371/journal.pone.0172900.
Li R, Yao Y, Gao P, Bu S. The therapeutic efficacy of curcumin vs. metformin in modulating the gut microbiota in NAFLD rats: a comparative study. Front Microbiol. 2021. https://doi.org/10.3389/fmicb.2020.555293.
Choudhury ST, Das N, Ghosh S, Ghosh D, Chakraborty S, Ali N. Vesicular (liposomal and nanoparticulated) delivery of curcumin: a comparative study on carbon tetrachloride–mediated oxidative hepatocellular damage in rat model. Int J Nanomedicine. 2016. https://doi.org/10.2147/IJN.S101886.
Lee HY, Kim SW, Lee GH, Choi MK, Jung HW, Kim YJ, Kwon HJ, Chae HJ. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complement Altern Med. 2016. https://doi.org/10.1186/s12906-016-1307-6.
Badria FA, Ibrahim AS, Badria AF, Elmarakby AA. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0134156.
Mendonça LM, da Silva Machado C, Teixeira CC, de Freitas LA, Bianchi MD, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology. 2013. https://doi.org/10.1016/j.neuro.2012.09.011.
Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009.
Zia A, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed Pharmacother. 2021. https://doi.org/10.1016/j.biopha.2020.111119.
Wang J, Wang C, Bu G. Curcumin inhibits the growth of liver cancer stem cells through the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. Exp Ther Med. 2018. https://doi.org/10.3892/etm.2018.5805.
Pricci M, Girardi B, Giorgio F, Losurdo G, Ierardi E, Di Leo A. Curcumin and colorectal cancer: From basic to clinical evidences. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21072364.
Xu XY, Meng X, Li S, Gan RY, Li Y, Li HB. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients. 2018. https://doi.org/10.3390/nu10101553.
Toydemir T, Kanter M, Erboga M, Oguz S, Erenoglu C. Antioxidative, antiapoptotic, and proliferative effect of curcumin on liver regeneration after partial hepatectomy in rats. Toxicol Ind Health. 2015. https://doi.org/10.1177/0748233712469658.
Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018. https://doi.org/10.1097/CEJ.000000000000042.
World Health Organization, WORLD HEALTH STATISTICS - monitoring health for the SDGs. World Heal Organ. 2016.
Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012. https://doi.org/10.4254/wjh.v4.i3.74.
Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002. https://doi.org/10.1053/jhep.2002.33638.
Ogbu UC, Arah OA. World Health Organization. Int Encycl Public Heal. 2016. https://doi.org/10.1016/B978-0-12-803678-5.00499-9.
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012. https://doi.org/10.1053/j.gastro.2011.12.061.
Osawa M, Akuta N, Suzuki F, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Arase Y, Suzuki Y, Ikeda K, Kumada H. Prognosis and predictors of hepatocellular carcinoma in elderly patients infected with hepatitis B virus. J Med Virol. 2017. https://doi.org/10.1002/jmv.24890.
Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014. https://doi.org/10.1002/hep.26986.
Galbraith JW, Franco RA, Donnelly JP, Rodgers JB, Morgan JM, Viles AF, Overton ET, Saag MS, Wang HE. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015. https://doi.org/10.1002/hep.27410.
Jacobson IM, Davis GL, El-Serag H, Negro F, Trépo C. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010. https://doi.org/10.1016/j.cgh.2010.06.032.
Elmore K, Nelson R, Gant Z, Jeffries C, Broeker L, Mirabito M, Roberts H. Data harmonization process for creating the national center for HIV/AIDS, viral hepatitis, STD, and TB prevention atlas. Public Health Rep. 2014. https://doi.org/10.1177/00333549141291s110.
Kanwal F, Kramer JR, Ilyas J, Duan Z, El‐Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014. https://doi.org/10.1002/hep.27095.
Vescovo T, Refolo G, Vitagliano G, Fimia GM, Piacentini M. Molecular mechanisms of hepatitis C virus–induced hepatocellular carcinoma. Clin Microbiol Infect. 2016. https://doi.org/10.1016/j.cmi.2016.07.019.
WATANABE S. Pathophysiology of nonalcoholic steatohepatitis. Juntendo Med. J. 2017. https://doi.org/10.14789/jmj.63.230.
Serfaty L, Lemoine M. Definition and natural history of metabolic steatosis: clinical aspects of NAFLD, NASH and cirrhosis. Diabetes Metab. 2008. https://doi.org/10.1016/S1262-3636(08)74597-X.
Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010. https://doi.org/10.1038/nrgastro.2010.100.
Dornas W, Schuppan D. Mitochondrial oxidative injury: a key player in nonalcoholic fatty liver disease. Am J Physiol - Gastrointest Liver Physiol. 2020. https://doi.org/10.1152/AJPGI.00121.2020.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016. https://doi.org/10.1002/hep.28431.
Mikolasevic I, Milic S, Wensveen TT, Grgic I, Jakopcic I, Stimac D, Wensveen F, Orlic L. Nonalcoholic fatty liver disease - a multisystem disease? World J Gastroenterol. 2016. https://doi.org/10.3748/wjg.v22.i43.9488.
Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation. 2019. https://doi.org/10.1097/TP.0000000000002484.
Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol. 2015. https://doi.org/10.4254/wjh.v7.i17.2080.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018. https://doi.org/10.1002/hep.29367.
Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA - J Am Med Assoc. 2020. https://doi.org/10.1001/jama.2020.2298.
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018. https://doi.org/10.1038/nrgastro.2017.109.
Yuan Y, Liu L, Chen H, Wang Y, Xu Y, Mao H, Li J, Mills GB, Shu Y, Li L, Liang H. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell. 2016. https://doi.org/10.1016/j.ccell.2016.04.001.
Saito T, Kuma A, Sugiura Y, Ichimura Y, Obata M, Kitamura H, Okuda S, Lee HC, Ikeda K, Kanegae Y, Saito I, Auwerx J, Motohashi H, Suematsu M, Soga T, Yokomizo T, Waguri S, Mizushima N, Komatsu M. Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-08829-3.
Tanaka S, Hikita H, Tatsumi T, Sakamori R, Nozaki Y, Sakane S, Shiode Y, Nakabori T, Saito Y, Hiramatsu N, Tabata K, Kawabata T, Hamasaki M, Eguchi H, Nagano H, Yoshimori T, Takehara T. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016. https://doi.org/10.1002/hep.28820.
Pawlotsky JM, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, Marra F, Puoti M, Wedemeyer H, European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series q European Association for the Study of the Liver*. J Hepatol. 2020.
Ganne-Carrié N, Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol. 2019. https://doi.org/10.1016/j.jhep.2018.10.008.
Meadows GG, Zhang H. Effects of alcohol on tumor growth, metastasis, immune response, and host survival. Alcohol Res Curr Rev. 2015.
Setshedi M, Wands JR, De La Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010. https://doi.org/10.4161/oxim.3.3.12288.
Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Brückner S, Zeissig S, Stephan AM, Wodarz N, Devière J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Völzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nöthen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015. https://doi.org/10.1038/ng.3417.
Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointest Liver Dis. 2013.
Wu HC, Santella R. The role of aflatoxins in hepatocellular carcinoma. Hepat Mon. 2012. https://doi.org/10.5812/hepatmon.7238.
Woo LL, Egner PA, Belanger CL, Wattanawaraporn R, Trudel LJ, Croy RG, Groopman JD, Essigmann JM, Wogan GN. Aflatoxin B1-DNA adduct formation and mutagenicity in livers of neonatal male and female B6C3F1 mice. Toxicol Sci. 2011. https://doi.org/10.1093/toxsci/kfr087.
Yu MW, Lien JP, Chiu YH, Santella RM, Liaw YF, Chen CJ. Effect of aflatoxin metabolism and DNA adduct formation on hepatocellular carcinoma among chronic hepatitis B carriers in Taiwan. J Hepatol. 1997. https://doi.org/10.1016/S0168-8278(97)80178-X.
Yang JD, Mohamed EA, Aziz AO, Shousha HI, Hashem MB, Nabeel MM, Abdelmaksoud AH, Elbaz TM, Afihene MY, Duduyemi BM, Ayawin JP. Gyedu A, Lohouès-Kouacou MJ, Ndam AWN, Moustafa EF, Hassany SM, Moussa AM, Ugiagbe RA, Omuemu CE, Anthony R, Palmer D, Nyanga AF, Malu AO, Obekpa S, Abdo AE, Siddig AI, Mudawi HMY, Okonkwo U, Kooffreh-Ada M, Awuku YA, Nartey YA, Abbew T, Awuku NA, Otegbayo JA, Akande KO, Desalegn HM, Omonisi AE, Ajayi AO, Okeke EN, Duguru MJ, Davwar PM, Okorie MC, Mustapha S, Debes JD, Ocama P, Lesi OA, Odeghe E, Bello R, Onyekwere C, Ekere F, Igetei R, Mah’moud MA, Addissie B, Ali HM, Gores GJ, Topazian MD, Roberts LR. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol. 2017. https://doi.org/10.1016/S2468-1253(16)30161-3.
Nasr M, Selima E, Hamed O, Kazem A. Targeting different angiogenic pathways with combination of curcumin, leflunomide and perindopril inhibits diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharmacol. 2014. https://doi.org/10.1016/j.ejphar.2013.11.022.
Acknowledgements
Nighat Gul and Arif Ahmed are grateful to University Grants Commission (UGC), New Delhi, India, for providing financial assistance to accomplish this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Cancer is the second major cause of death due to non-communicable diseases worldwide.
• Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide.
• Curcuma longa plant has gained widespread attention in recent years because of its multifunctional properties as an antioxidant, anti-inflammatory, antimicrobial, and anticancer agent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gull, N., Arshad, F., Naikoo, G.A. et al. Recent Advances in Anticancer Activity of Novel Plant Extracts and Compounds from Curcuma longa in Hepatocellular Carcinoma. J Gastrointest Canc 54, 368–390 (2023). https://doi.org/10.1007/s12029-022-00809-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00809-z