Abstract
Purpose
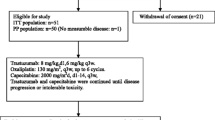
In the ToGA trial for HER2-positive advanced gastric cancer, cisplatin plus fluoropyrimidine was given for 6 cycles; trastuzumab was given until disease progression. However, there is a lack of real-life data about trastuzumab maintenance after 6 cycle chemotherapy. This study aims to present real-life data of trastuzumab ± capecitabine maintenance after 6 cycles of platinum, fluoropyrimidine, and trastuzumab in non-progressive patients.
Methods
This is a retrospective multicenter study of the Turkish Oncology Group. A total of 35 HER2-positive, inoperable locally advanced, recurrent, or metastatic gastric adenocarcinoma patients being non-progressive at the end of 6 cycle chemotherapy and being given trastuzumab ± capecitabine as maintenance treatment were included from sixteen oncology centers. Baseline characteristics, objective tumor responses, progression free and overall survival data, and toxicities were determined.
Results
About 68% of the patients were given CF, and 32% were given FOLFOX with trastuzumab as the first-line treatment. The best response in 6 cycle chemotherapy was complete 8 (22%), partial 24 (68%), and stable disease 3 (8%). All patients had trastuzumab maintenance (median cycle 13; range 7–51), and 49% of the patients had capecitabine with trastuzumab (median capecitabine cycle 6; range 2–30). The median PFS of the patients was 12.0 months (95% CI 10.3–13.7), and median OS was 17.4 months (95% CI 15.2–19.5). There were 2 patients with grade 1 cardiotoxicity.
Conclusion
Trastuzumab maintenance ± capecitabine after 6 cycles of trastuzumab plus combined chemotherapy treatment revealed efficacy and safety in non-progressive HER2-positive advanced gastric cancer.

Similar content being viewed by others
Data Availability
The data is available.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. https://doi.org/10.1016/s0140-6736(10)61121-x.
Koopman T, Smits MM, Louwen M, Hage M, Boot H, Imholz AL. HER2 positivity in gastric and esophageal adenocarcinoma: clinicopathological analysis and comparison. J Cancer Res Clin Oncol. 2015;141(8):1343–51. https://doi.org/10.1007/s00432-014-1900-3.
Fornaro L, Vivaldi C, Parnofiello A, Ugolini C, Aprile G, De Maglio G, et al. Validated clinico-pathologic nomogram in the prediction of HER2 status in gastro-oesophageal cancer. Br J Cancer. 2019;120(5):522–6. https://doi.org/10.1038/s41416-019-0399-4.
Liang Z, Zeng X, Gao J, Wu S, Wang P, Shi X, et al. Analysis of EGFR, HER2, and TOP2A gene status and chromosomal polysomy in gastric adenocarcinoma from Chinese patients. BMC Cancer. 2008;8:363. https://doi.org/10.1186/1471-2407-8-363.
Ter Veer E, Creemers A, de Waal L, van Oijen MGH, van Laarhoven HWM. Comparing cytotoxic backbones for first-line trastuzumab-containing regimens in human epidermal growth factor receptor 2-positive advanced oesophagogastric cancer: a meta-analysis. Int J Cancer. 2018;143(2):438–48. https://doi.org/10.1002/ijc.31325.
Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68. https://doi.org/10.1186/s12885-016-2092-9.
Soularue E, Cohen R, Tournigand C, Zaanan A, Louvet C, Bachet JB, et al. Efficacy and safety of trastuzumab in combination with oxaliplatin and fluorouracil-based chemotherapy for patients with HER2-positive metastatic gastric and gastro-oesophageal junction adenocarcinoma patients: a retrospective study. Bull Cancer. 2015;102(4):324–31. https://doi.org/10.1016/j.bulcan.2014.08.001.
Li Q, Lv M, Jiang H, Wang Y, Yu S, Li W, et al. A prospective observational study on the optimal maintenance strategy in HER2-positive advanced gastric cancer treated with trastuzumab-based therapy. J Cancer Res Clin Oncol. 2020;146(1):287–95. https://doi.org/10.1007/s00432-019-03060-5.
Ilhan-Mutlu A, Taghizadeh H, Beer A, Dolak W, Ba-Ssalamah A, Schoppmann SF, et al. Correlation of trastuzumab-based treatment with clinical characteristics and prognosis in HER2-positive gastric and gastroesophageal junction cancer: a retrospective single center analysis. Cancer Biol Ther. 2018;19(3):169–74. https://doi.org/10.1080/15384047.2017.1414759.
Lopez A, Chen H-C, Harada K, Murphy MAB, Rogers JE, Thomas I et al. Maintenance therapy with trastuzumab +/- fluoropyrimidine in non-progressive HER-2 positive advanced gastroesophageal cancer patients after first-line chemotherapy. J Clin Oncol. 2018;36(15_suppl):e16071-e. https://doi.org/10.1200/JCO.2018.36.15_suppl.e16071.
Ryu MH, Yoo C, Kim JG, Ryoo BY, Park YS, Park SR, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer. 2015;51(4):482–8. https://doi.org/10.1016/j.ejca.2014.12.015.
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110(5):1163–8. https://doi.org/10.1038/bjc.2014.18.
Chua C, Tan IB, Yamada Y, Rha SY, Yong WP, Ong WS, et al. Phase II study of trastuzumab in combination with S-1 and cisplatin in the first-line treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol. 2015;76(2):397–408. https://doi.org/10.1007/s00280-015-2811-y.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Author information
Authors and Affiliations
Contributions
MG, EA, and FÇŞ designed the study. MG and EA analyzed the data and wrote the manuscript. FÇŞ did final review. AS, SU, AGD, SŞ, TŞ, CE, MANŞ, ABŞ, EÇ, DCG, SK, YE, DU, NST, NÜ, HYÇ, AD, RA, NK, ST, MA, and MK contributed the data and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval, Consent to Participate, and Consent for Publication
Approval of ethical committee was obtained from Ankara University Faculty of Medicine Ethics Committee in compliance with Helsinki Declaration (Decision number: 03-196-19).
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gürbüz, M., Akkuş, E., Sakin, A. et al. Trastuzumab ± Capecitabine Maintenance After the First-Line Treatment of HER2-Positive Advanced Gastric Cancer: Retrospective Observational Real-Life Data of Turkish Oncology Group. J Gastrointest Canc 53, 282–288 (2022). https://doi.org/10.1007/s12029-021-00594-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-021-00594-1