Abstract
Background
Patients who require readmission to an intensive care unit (ICU) after transfer to a lower level of care (“bounceback”) suffer from increased mortality and longer hospital stays. We aimed to create a multifaceted standardized transfer process for patients moving from the neurointensive care unit (neuro-ICU) to a lower level of care. We hypothesized that this process would lead to improvement in provider-rated safety and a decreased rate of bouncebacks to the neuro-ICU after transfer.
Methods
The study took place at the Hospital of the University of Pennsylvania from October 2018 to October 2020. A standardized five-step transfer process was created and implemented for transferring patients from the neuro-ICU to a lower level of care. Patient care providers completed a survey before and after implementation of the protocol to assess a variety of components related to safety concerns when transferring patients. The rate of bouncebacks pre and post intervention was calculated by using a two-sample Wilcoxon rank-sum test, and disposition at discharge was calculated by using Fisher’s exact test.
Results
Of the 1176 total patient transfers out of the neuro-ICU, 29 patients bounced back within 48 h. The average age of patients who bounced back was 63.3 years old, with a similar distribution among men and women. The most common reason for bounceback was respiratory distress, followed by cardiac arrhythmia, stroke, and sepsis. Implementation of the standardized process led to a decrease in provider-rated concern of overall safety (5 to 3, p = 0.008). There was improvement in transfer delays due to bed availability (3 to 4.5, p = 0.020), identification of high-risk patients (5 to 6, p = 0.021), patient assignment to the appropriate level of care (5 to 6, p = 0.019), and use of the electronic medical record handoff indicator (5 to 6, p = 0.003). There was no statistically significant difference in terms of patient bounceback rate after implementation of the process (2.4% vs. 2.5%, p = 1.00) or patient disposition at discharge (p = 0.553).
Conclusions
Patients who bounceback to the neuro-ICU within 48 h had an increased length of hospital stay, had an increased length of ICU stay, and were more likely to be intubated for more than 96 h. Implementation of a standardized five-step transfer process from the neuro-ICU to a lower level of care resulted in improvement in multiple provider-rated safety outcomes and identification of high-risk patients but led to no difference in the patient bounceback rate or patient disposition at discharge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An adverse event is an injury caused by medical conduct that can lead to prolonged patient hospitalization, disability, or mortality [1, 2]. There have been between 44,000 and 98,000 patient deaths per year in US hospitals caused by medical errors [2, 3]. Poor communication is a contributing factor in more than 60% of reported hospital adverse events [4, 5], occurs frequently during transitions of care, and is preventable. Audits of reports for patients transferred out of the intensive care unit (ICU) at a multidisciplinary teaching hospital found that 62% of reports had at least one error, with 19% resulting in either serious or critical errors [6]. Furthermore, patients admitted by cross-covering residents who then get transferred to a different resident end up with more tests in the hospital and longer hospital stays [1].
Moreover, patients who require readmission to an ICU after transfer to a lower level of care have longer hospital stays and increased mortality [7, 8]. Most studies related to patient handoffs and ICU readmission are from medical or surgical ICUs and have limited focus on patients specifically with neurologic disease [9,10,11]. Coughlin et al. [9] identified the most common reasons for patients to bounce back specifically to a neurointensive care unit (neuro-ICU) after being transferred to a lower acuity level of care, including respiratory distress, sepsis/hypotension, need for additional nursing monitoring, cardiac issues, and hypertension.
Standardized handoff processes and checklists can help reduce errors and adverse events. For instance, use of the standardized SBAR (Situation, Background, Assessment, Recommendation) handoff system decreased adverse events in a hospital from 90 to 40 per 1,000 patient-days and decreased adverse drug events from 30 to 18 per 1,000 patient-days [12,13,14]. Another standardized handoff system called I-PASS (Illness severity, Patient summary, Action list, Situation awareness and contingency plans, and Synthesis by receiver) was created for use at an academic hospital in California [15]. This initiative to create a standardized handoff for pediatric patients transitioning from the cardiovascular ICU to the acute care unit resulted in significantly improved transfer efficacy, safety culture scores, and satisfaction for both families and medical providers. In previous work at our own institution, Coughlin et al. [9] created a standardized checklist known as a “green sheet” that identified neurology patients at high risk of bouncing back to the neuro-ICU. The sheet required providers to check risk factors a patient had that would increase their risk of bounceback to the neuro-ICU, including if they required intubation for more than 4 days, reintubation during admission, antibiotic changes, intravenous (IV) antihypertension medications, IV pressors, treatment of multiple seizures, or treatment of cardiac arrhythmias. If any of these risk factors were present, the green sheet was hung over the patient’s bed and the receiving physician plus nurse would evaluate the patient within 1 h of transfer. Additionally, the respiratory therapist would evaluate the patient within 2 h of transfer to discern if any respiratory issues needed addressing. The approach of having multiple providers evaluate the patient in a timely manner was aimed at preventing the most common complications leading to bounceback. Their intervention was implemented in three, 3-month stages: stage 1, only physicians used the checklist; stage 2, nurses began using the checklist; and stage 3, respiratory therapists were added. Use of this tool resulted in decreased rate of patient bouncebacks from a baseline of 6.7% prior to intervention to 2.8% in the final stage of implementation (p for trend = 0.09). The intervention also resulted in improved provider satisfaction. However, this checklist approach was a passive process to identify high-risk patients. We aimed to build on this structure by creating multiple checkpoints to prevent high-risk patients from being prematurely transferred and ensuring a more standardized handoff system to prepare the accepting providers for high-risk patients.
The purpose of our study was to create a multifaceted and standardized transfer process for patients moving from the neuro-ICU to a lower acuity unit (floor or intermediate critical care unit). We hypothesized that use of the standardized transfer process would improve provider-rated safety regarding patient transfers and reduce the rate of bouncebacks to the neuro-ICU.
Methods
Study Approval
The Hospital of the University of Pennsylvania Institutional Review Board reviewed the study and determined that it did not constitute human subject or clinical investigation research. Full review was waived under a quality improvement exemption. All authors complied with Health Insurance Portability and Accountability Act regulations.
Setting
The study took place at the Hospital of the University of Pennsylvania in Philadelphia, the flagship quaternary care center of the academic health system. The hospital is equipped with a 22-bed neuro-ICU that covers both neurology and neurosurgery patients. The neuro-ICU and non-ICU neurology inpatient services (stroke and general ward services) are located in separate buildings within the larger hospital system. Neuro-ICU providers have a geographically distinct work space from the accepting neurology provider teams. Patients on the stroke and general ward service reside either in the intermediate critical care unit or the floor (the lowest acuity level of care). The criteria by which patients can be transferred from the neuro-ICU to the intermediate critical care unit include but are not limited to the following: require less than 24 h of hourly vital checks, maintain respiratory stability on continuous positive airway pressure without need for mechanical ventilation, maintain hemodynamic stability without pressor requirement, and require benzodiazepines for treatment of seizures without the need for continuous IV anesthetic. Although the above criteria exist, the ultimate decision as to whether a patient is deemed stable for transfer out of the neuro-ICU is at the discretion of the neurocritical care attending caring for the patient.
Survey/Standardized Process
We sent a preintervention survey to the neurology residents, attendings, ICU fellows, and floor and ICU nurses to assess their perceived safety ratings regarding transferring patients from the neuro-ICU to a lower acuity unit. The survey was conducted via REDCap and sent via email to providers. Providers were asked before and after the intervention to rate on a 10-point Likert scale (1 = strongly disagree, 10 = strongly agree) if transferring patients from the neuro-ICU to neurology services resulted in significant safety issues. Additionally, providers were asked to rate using a 10-point Likert scale (1 = needs significant improvement, 10 = need no improvement) which factors related to patient transfers needed improvement, specifically, verbal communication between providers/nurses, written documentation, identification of high-risk patients, overnight transfers, notification of patient arriving to the floor, assigning the appropriate level of care, and patient stability on transfer.
The framework for patient transfers created by Coughlin et al. [9] included identification of high-risk patient transfers, verbal handoffs between providers, text alerts after patient transfers, and evaluation of patients on arrival. We built on this framework by standardizing each of these steps and adding additional steps that addressed the weakest areas of the transfer process on the basis of the preintervention survey. On October 28, 2019, we implemented this new standardized five-step process to transfer patients from the neuro-ICU to a lower acuity unit (Fig. 1). The process entailed the transferring team discussing potential patient transfers, completing a verbal handoff to the accepting provider by a specific time point, and writing a standardized note (see Supplemental Materials) and the accepting provider receiving a text alert notification of patient transfer and arrival to unit and evaluating patients after transfer. After implementation of the standardized transfer process, a postintervention survey was sent to neurology providers to assess safety rating and satisfaction with the new protocol. Providers were trained about the new standardized process at one of our weekly morbidity and mortality conferences. An outline of the process was also sent to providers via email to use as a reference when needed.
Analysis
We then conducted a retrospective analysis of patients who bounced back after transfer. Similar to the prior study by Coughlin et al. [9], bounceback was defined as either an unplanned return to the neuro-ICU or an unplanned transfer from a floor bed to an intermediate critical care unit bed within 48 h of transfer from the neuro-ICU. We included all patients transferred to the neurology service and excluded patients from the neurosurgical services. Patient transfer data, including discharge diagnosis, were obtained by automated extraction from the electronic medical record. Manual chart review to confirm patient level of care, discharge diagnosis, and reason for bounceback was performed by a senior neurology resident and hospital senior improvement advisor. Pre- and postintervention Likert scale survey data were compared by using a t-test in Stata. Average hospital length of stay and ICU length of stay for pre- and postintervention and bounceback and no bounceback patients were compared by using a two-sample Wilcoxon rank-sum test. Patient discharge disposition and ventilatory status for pre- and postintervention and bounceback and no bounceback patients were compared by using Fisher’s exact test.
Results
Survey Data
The preintervention survey was completed by 109 of 289 (38%) total providers: 22 of 40 neurology residents, 9 of 18 neurology attendings, 6 of 14 neurocritical care attendings, 28 of 104 ICU nurses, 36 of 103 floor nurses, 3 of 5 neurocritical care nurse practitioners, and 5 of 5 neurocritical care fellows. The postintervention survey was completed by 55 of 288 (19%) total providers: 26 of 42 neurology residents, 4 of 18 neurology attendings, 4 of 14 neurocritical care attendings, 14 of 104 ICU nurses, 7 of 100 floor nurses, 0 of 5 neurocritical care advanced care providers, and 0 of 5 neurocritical care fellows. The median Likert scale rating for safety concerns decreased from 5 preintervention to 3 post intervention (p = 0.008; Table 1). For the postintervention survey, 66% of participants agreed that the new standardized transfer process improved patient care, 30% were not aware the process existed, and 4% did not believe there was improvement in patient care. Moreover, 40% of postintervention survey participants agreed that the automated text alert system at time of bed assignment and transfer decreased the number of patients arriving to the floor without the primary physician being aware (39% were unsure, 11% did not agree).
Across all providers in the postintervention arm, there was statistically significant improvement in the following categories related to patient transfers: delays in transfers due to bed availability (3–4.5, p = 0.020), identification of patients at high risk for bounceback (5–6, p = 0.021), assignment to the appropriate level of care (5–6, p = 0.019), and use of the electronic medical record handoff indicator (5 to 6, p = 0.003), as seen in Table 1. There was no statistically significant difference in the following categories: verbal communication between providers (5–6, p = 0.056), patient stability on transfer (6–5.5, p = 0.947), verbal communication between nurses (5–6, p = 0.485), written communication (5–5, p = 0.417), overnight transfers (4–5, p = 0.073), and notification of patient arrival to the unit (5–6.5, p = 0.162).
Intervention Data
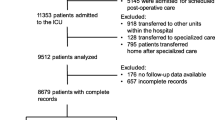
There was a total of 1176 neurology patient transfers from the neuro-ICU to a lower-level acuity unit between October 2018 and October 2020. Among those transfers, 663 occurred prior to the intervention and 513 occurred after the intervention (Fig. 2). Patient demographics pre and post intervention were similar (Table 2). The difference in rate of bounceback pre and post intervention was not statistically significant (2.4% vs. 2.5%, p = 1.00) as seen in Table 2. The median length of hospital stay was shorter for the preintervention group (6 vs. 8 days, p = 0.001), as was the median ICU length of stay (47 vs. 49 h, p = 0.144). The percentage of patients intubated for more than 96 h was similar between pre- and postintervention groups (15.5% vs. 19.9%, p = 0.053). There was no statistically significant difference in reason for bounceback in the pre- and postintervention groups (Table 3).
Demographic data for patients who bounced back and those who did not bounce back to the neuro-ICU were similar (Table 4). The cohort of patients who bounced back compared with those who did not bounce back to the neuro-ICU had a longer median length of hospital stay (17 vs. 7 days, p < 0.001), had a longer median length of ICU stay (216 vs. 46 h, p < 0.001), and were more likely to be intubated for more than 96 h (41.4% vs. 16.8%, p = 0.002), as seen in Table 4.
Discussion
Implementation of a standardized five-step transfer process resulted in provider-rated improvement in overall safety regarding to transferring patients from a neuro-ICU to a lower level of care. Specific aspects of patient transfers that were rated as the most significant improvements included delays in transfer due to bed availability, identification of high-risk patients, assignment of patients to the appropriate level of care, and use of the electronic medical record handoff indicator. Despite the fact that an automated text alert system was implemented, there was no statistically significant improvement in provider-rated safety regarding notification of patient arrival to the unit. A potential reason for this discrepancy is that only a single provider is notified, and thus all providers involved in the transfer process may not benefit from this notification. Additionally, there was no significant difference in provider-rated safety regarding verbal or written communication, despite the implementation of a standardized verbal and written handoff. The written note itself might have been perceived as redundant documentation, and the compliance with this part of the process might have been limited. Moreover, providers were likely already performing some form of verbal handoff prior to the implementation of this process as part of prior work by Coughlin et al. [9]. Although there was no significant improvement with provider-rated safety in terms of overnight transfers and verbal communication between nurses, the standardized transfer process did not directly intervene on these measures.
Another limitation with the survey component was that a fair number of providers did not respond to the postintervention survey (54 fewer participants in the postintervention survey than in the preintervention survey), and, notably, no neurocritical care fellows responded to the postintervention survey. The exact reason for response dropout cannot be elucidated and is likely multifactorial. We suspect that factors related to COVID-19 could have played a role. More specifically, health care providers during this time had increased clinical responsibilities, higher rates of burnout, and decreased face-to-face interactions, all of which could have hindered motivation to complete the postintervention survey [18].
It should be noted that demographic information for respondents was limited to provider role in an attempt to maintain anonymity. Moreover, given that the proportion of providers pre and post intervention was different, the pre- and postintervention populations were not the exact same individuals. However, the individuals surveyed came from the same groups of people, both pre and post intervention (i.e., neurology residents, nurses, etc.)
The fact that 30% of surveyed participants were not aware the process existed suggests that compliance with the protocol might have been a reason bounceback rates pre and post intervention were not significantly different. Also, the study took place over three academic cycles; thus, some new providers might not have been familiar with the process and some providers involved in the preintervention arm were not available for follow-up for the postintervention survey. Moreover, providers’ roles might have changed from one year to the next, which might have also influenced the results of the survey. Perhaps more frequent intermittent education about the protocol throughout the year would increase compliance.
Patients in our cohort who bounced back to the neuro-ICU had longer length of hospital stay and had a longer length of ICU stay, which was similar to other data regarding bouncebacks [1, 2, 10]. There were a higher percentage of patients intubated for more than 96 h in those who bounced back compared with those who did not bounce back; however, there was no statistically significant difference in mortality between these two groups. Although there was no difference in mortality, prolonged intubation and length of stay portend increased morbidity, including increased risk of laryngeal injury [16].
The definition of bounceback to an ICU is not standardized across all studies; some consider readmission to an ICU at any point during the hospitalization a bounceback as opposed to within 48 h of transfer, as used in our study. Depending on the definition, the average readmission rates in medical and surgical ICUs range between 4 and 14%, with an average of 7% [10, 17]. Reported readmission rates in neuro-ICUs are less well studied but range between 2.8% and 21% [9, 11]. It should be noted that the baseline bounceback rate at our institution prior to the current intervention was relatively low at 2.4%, likely as a result of the previous “green sheet” intervention by Coughlin et al. [9]. The prior multidisciplinary approach to evaluating high-risk patients on arrival to a lower acuity unit likely helped maintain the low bounceback rate. Starting at a relatively low bounceback rate might have been a contributing reason as to why we did not observe significant improvement in the bounceback rate with our current intervention [9]. There may be a floor effect limiting further reductions, as some bouncebacks are ultimately neither predictable nor preventable. Similar to the study by Coughlin et al. [9], the most common reason for patients to bounce back, both pre and post intervention, was respiratory distress. There were a higher percentage of patients in the preintervention group with respiratory distress than in the postintervention group, although this was not statistically significant (56.3% vs. 53.9%, p = 0.541).
The postintervention cohort had a longer median length of hospital stay compared with the preintervention group. A likely contributing factor was the COVID-19 pandemic, which took effect during this postintervention period. None of the patients who bounced back in the postintervention group were diagnosed with COVID-19 during their hospitalization. However, the limited capacity of nursing/rehabilitation facilities and the need for negative testing prior to discharge during the peak of the pandemic likely contributed to delayed discharges with longer duration of hospital stays [19]. During the postintervention period, the hospital also experienced fewer outside hospital transfers, elective admissions, and stroke cases, which might have resulted in an increased availability of lower acuity unit floor beds. The increased availability of lower acuity beds could have explained why providers noted less of a delay for ICU to floor transfers due to bed availability. The single-center design of the study is an additional limitation, and perhaps future research involving multiple centers would provide more insight into the optimization of patient transfers as well as the impact of transfers amid the COVID-19 pandemic.
Conclusions
Implementation of our standardized five-step transfer process from the neuro-ICU to a lower level of care resulted in overall improved provider-rated safety outcomes. Specific aspects of patient transfers were rated as improved, including delays in transfers due to bed availability, identification of high-risk patients, patient assignment to the appropriate level of care, and use of the electronic medical record handoff indicator. Patients who bounced back to the ICU had longer lengths of hospital and ICU stays, and a higher percentage required prolonged intubation, thus making this an important issue to study further. There was no difference in the rate of bounceback pre and postintervention although limitations regarding provider awareness of the process and the COVID-19 pandemic might have contributed to these findings. The standardized five-step transfer process may be used as a stepping stone for further research to optimize transfers for patients with critical neurologic diseases.
References
Solet DJ, Norvell JM, Rutan GH, Frankel RM. Lost in translation: challenges and opportunities in physician-to-physician communication during patient handoffs. Acad Med. 2005;80(12):1094–9.
Kohn LT, Corrigan JM, Donaldson MS, McKenzie D (2000) To err is human: building a safer healthcare system, in committee on quality and healthcare in America, Institute of Medicine. Washington, DC: National Academy Press.
Volpp K, Grande D. Residents’ suggestions for reducing errors in teaching hospitals. N Engl J Med. 2003;348:9.
The Joint Commission. Sentinel event data: root causes by event type 2004–2014.
Sectish TC, Starmer AJ, Landrigan CP, Spector ND. Establishing a multisite education and research project requires leadership, expertise, collaboration, and an important aim. Pediatrics. 2010;126:4.
Perren A, Conte P, De Bitonti N, Limoni C, Merlani P. From the ICU to the ward: cross-checking of the physician’s transfer report by intensive care nurses. Intensive Care Med. 2008;34(11):2054–61. https://doi.org/10.1007/s00134-008-1138-0.
Alban RF, Nisim AA, Ho J, Nishi GK, Shabot MM. Readmission to surgical intensive care increases severity-adjusted patient mortality. J Trauma. 2006;60(5):1027–31.
Cooper GS, Sirio CA, Rotondi AJ, Shepardson LB, Rosenthal GE. Are readmissions to the intensive care unit a useful measure of hospital performance? Med Care. 1999;37(4):399–408.
Coughlin DG, Kumar MA, Patel NN, Hoffman RL, Kasner SE. Preventing early bouncebacks to the neurointensive care unit: a retrospective analysis and quality improvement pilot. Neurocrit Care. 2018;28(2):175–83. https://doi.org/10.1007/s12028-017-0446-z.
Gold CA, Mayer SA, Lennihan L, et al. Unplanned transfers from hospital wards to the neurological intensive care unit. Neurocrit Care. 2015;23:159–65. https://doi.org/10.1007/s12028-015-0123-z.
Murray NM, Joshi AN, Kronfeld K, Hobbs K, Bernier E, Hirsch KG, Gold CA. A standardized checklist improves the transfer of stroke patients from the neurocritical care unit to hospital ward. Neurohospitalist. 2020;10(2):100–8. https://doi.org/10.1177/1941874419873810.
Pope BB, Rodzen L, Spross G. Raising the SBAR: How better communication improves patient outcomes. Nursing. 2008;38(3):41–3.
Oakes SL, Gillespie SM, Ye Y, Finley M, Russell M, Patel NK, Espino D. Transitional care of the long-term care patient. Clin Geriatr Med. 2011;27(2):259–71.
Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32(3):167–75.
Sheth S, McCarthy E, Kipps AK, Wood M, Roth SJ, Sharek PJ, Shin AY. Changes in efficiency and safety culture after integration of an I-PASS-supported handoff process. Pediatrics. 2016;137(2):e20150166. https://doi.org/10.1542/peds.2015-0166.
Tadié JM, Behm E, Lecuyer L, Benhmamed R, Hans S, Brasnu D, Diehl JL, Fagon JY, Guérot E. Post-intubation laryngeal injuries and extubation failure: a fiberoptic endoscopic study. Intensive Care Med. 2010;36(6):991–8. https://doi.org/10.1007/s00134-010-1847-z.
Rosenberg AL, Watts C. Patients readmitted to ICUs* : a systematic review of risk factors and outcomes. Chest. 2000;118(2):492–502. https://doi.org/10.1378/chest.118.2.492.
Sharma MK, Anand N, Singh P, et al. Researcher burnout: An overlooked aspect in mental health research in times of COVID-19. Asian J Psychiatr. 2020;54: 102367. https://doi.org/10.1016/j.ajp.2020.102367.
Olanipekun T. The impact of COVID-19 testing on length of hospital stay and patient flow in hospitals. J Community Hosp Intern Med Perspect. 2021;11(2):180–3. https://doi.org/10.1080/20009666.2020.1866249.
Acknowledgements
Jeffrey Rohrbach, MSN (University of Pennsylvania Health System, Philadelphia, PA, USA), collected data.
Funding
No funding was provided for the project.
Author information
Authors and Affiliations
Contributions
CLN: conception and design, acquisition of data, analysis/interpretation of data, drafting of the initial article, revising the article, formalizing the final draft, final approval. LS: conception and design, acquisition of data, analysis/interpretation of data, revising the article, final approval. LJG: conception and design, acquisition of data, analysis/interpretation of data, revising the article, final approval. BY: acquisition of data, revising the article, final approval. JF: acquisition of data, revising the article, final approval. BG: acquisition of data, revising the article, final approval. PD: acquisition of data, revising the article, final approval. SEK: conception and design, acquisition of data, analysis/interpretation of data, revising the article, final approval. MAK: conception and design, acquisition of data, analysis/interpretation of data, drafting of the article, revising the article, final approval.
Corresponding author
Ethics declarations
Conflicts of interest
Scott E. Kasner receives grant support from WL Gore and Associates, Bristol-Myers Squibb, Genentech, Medtronic; and consulting fees from Bristol-Myers Squibb, Medtronic, Abbvie, Abbott, and AstraZeneca. No other authors have conflicts of interest or financial disclosures.
Ethical approval/informed consent
The authors adhered to ethical guidelines. The Hospital of the University of Pennsylvania Institutional Review Board reviewed the study and determined that it did not constitute human subject or clinical investigation research. Full review was waived under a quality improvement exemption.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nathan, C.L., Stein, L., George, L.J. et al. Standardized Transfer Process for a Neurointensive Care Unit and Assessment of Patient Bounceback. Neurocrit Care 36, 831–839 (2022). https://doi.org/10.1007/s12028-021-01385-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01385-z