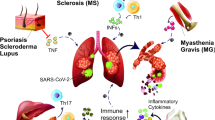
Abstract
Background
Routine use of immunosuppressive agents in systemic lupus erythematosus (SLE) patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) potentially increases the risk of adverse outcomes. belimumab, a monoclonal antibody for the treatment of SLE, remains untested for its specific impact on coronavirus disease 2019 (COVID-19) symptoms in these patients. Here, this research investigated the effect of belimumab on COVID-19 symptoms in SLE patients infected with SARS-CoV-2.
Methods
This study enrolled SLE patients who underwent treatment with belimumab. After thorough screening based on the inclusion and exclusion criteria, data pertaining to COVID-19 for both the participants and their cohabitants were obtained through telephone follow-up. The potential impact of belimumab on COVID-19 was evaluated by comparing COVID-19 symptoms and medication use across various groups to investigate the association between belimumab treatment and COVID-19 in SLE.
Results
This study involved 123 SLE patients, of whom 89.4% tested positive for SARS-CoV-2. Among cohabitants of SLE patients, the SARS-CoV-2 positive rate was 87.2% (p = 0.543). Patients treated with belimumab exhibited a lower incidence of multiple COVID-19 symptoms than their cohabitating counterparts (p < 0.001). This protective effect was found to be partially related to the time of last belimumab administration. Among those with COVID-19, 30 patients opted to discontinue their anti-SLE drugs, and among them, 53% chose to discontinue belimumab. Discontinuing drugs did not increase the risk of hospitalization due to SARS-CoV-2 infection.
Conclusion
This study concluded that treatment with belimumab did not increase susceptibility to COVID-19 and beneficially alleviated the symptoms of COVID-19.

Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Anka AU, Tahir MI, Abubakar SD, et al. Coronavirus Disease 2019 (COVID-19): an overview of the immunopathology, serological diagnosis and management. Scand J Immunol. 2021;93(4):e12998. https://doi.org/10.1111/sji.12998. PubMed PMID: 33190302; PubMed Central PMCID: PMCPMC7744910. eng.
(JHU). tCfSSaECaJHU. COVID-19 Dashboard. 2023.
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet (London England). 2020;395(10224):565–74. doi: 10.1016/s0140-6736(20)30251-8. PubMed PMID: 32007145; PubMed Central PMCID: PMCPMC7159086. eng.
Ge H, Wang X, Yuan X et al. The epidemiology and clinical information about COVID-19. European journal of clinical microbiology & infectious Diseases: official publication of the European Society of Clinical Microbiology. 2020;39(6):1011–9. https://doi.org/10.1007/s10096-020-03874-z. PubMed PMID: 32291542; PubMed Central PMCID: PMCPMC7154215. eng.
Leppkes M, Knopf J, Naschberger E, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. https://doi.org/10.1016/j.ebiom.2020.102925. PubMed PMID: 32745993; PubMed Central PMCID: PMCPMC7397705. eng.
Singh J, Herrmann I, Mahajan A, et al. A pleomorphic puzzle: heterogeneous pulmonary vascular occlusions in patients with COVID-19. Int J Mol Sci. 2022;23(23). https://doi.org/10.3390/ijms232315126. PubMed PMID: 36499449; PubMed Central PMCID: PMCPMC9739020. eng.
Kiriakidou M, Ching CL. Systemic Lupus Erythematosus. Ann Intern Med. 2020;172(11):Itc81–itc96. https://doi.org/10.7326/aitc202006020. PubMed PMID: 32479157; eng.
Akiyama S, Hamdeh S, Micic D, et al. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune Diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80(3):384–91. https://doi.org/10.1136/annrheumdis-2020-218946. PubMed PMID: 33051220; PubMed Central PMCID: PMCPMC7554412. eng.
Mathian A, Mahevas M, Rohmer J, et al. Clinical course of coronavirus Disease 2019 (COVID-19) in a series of 17 patients with systemic Lupus Erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020;79(6):837–9. https://doi.org/10.1136/annrheumdis-2020-217566. PubMed PMID: 32332072; eng.
Wallace B, Washer L, Marder W, et al. Patients with lupus with COVID-19: University of Michigan experience. Ann Rheum Dis. 2021;80(3):e35. https://doi.org/10.1136/annrheumdis-2020-217794. PubMed PMID: 32475835; eng.
Fortuna G, Brennan MT. Systemic Lupus Erythematosus: epidemiology, pathophysiology, manifestations, and management. Dental Clin N Am. 2013;57(4):631–55. https://doi.org/10.1016/j.cden.2013.06.003. PubMed PMID: 24034070; eng.
Bag-Ozbek A, Hui-Yuen JS. Emerging B-Cell therapies in systemic Lupus Erythematosus. Ther Clin Risk Manag. 2021;17:39–54. https://doi.org/10.2147/tcrm.S252592. PubMed PMID: 33488082; PubMed Central PMCID: PMCPMC7814238. eng.
van Vollenhoven RF, Petri MA, Cervera R et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Annals of the rheumatic diseases. 2012;71(8):1343-9. doi: 10.1136/annrheumdis-2011-200937. PubMed PMID: 22337213; PubMed Central PMCID: PMCPMC3396451 Genome Sciences and GlaxoSmithKline. MAP and RC have received payment for board membership and consultancy from Human Genome Sciences and GlaxoSmithKline. DAR, BNJ and CSK are employed by and own stock in GlaxoSmithKline. ZJZ and WF are employed by and own stock in Human Genome Sciences. eng.
Strand V, Levy RA, Cervera R, et al. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic Lupus Erythematosus from the randomised controlled BLISS trials. Ann Rheum Dis. 2014;73(5):838–44. https://doi.org/10.1136/annrheumdis-2012-202865. PubMed PMID: 23524886; PubMed Central PMCID: PMCPMC3995218. eng.
Spihlman AP, Gadi N, Wu SC, et al. COVID-19 and systemic Lupus Erythematosus: Focus on Immune response and therapeutics. Front Immunol. 2020;11:589474. https://doi.org/10.3389/fimmu.2020.589474. PubMed PMID: 33193418; PubMed Central PMCID: PMCPMC7661632. eng.
Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic Disease in Hubei Province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2(9):e557–64. https://doi.org/10.1016/s2665-9913(20)30227-7. PubMed PMID: 32838309; PubMed Central PMCID: PMCPMC7333992. eng.
Boedecker-Lips SC, Claßen P, Kraus D, et al. Belimumab is not associated with COVID-19 mRNA vaccination failure in systemic Lupus Erythematosus. Rheumatology (Oxford). 2023;62(3):e34–5. https://doi.org/10.1093/rheumatology/keac459. PubMed PMID: 35972412; PubMed Central PMCID: PMCPMC9384795. eng.
Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80(10):1322–9. https://doi.org/10.1136/annrheumdis-2021-220626. PubMed PMID: 34362747; PubMed Central PMCID: PMCPMC8350975. eng.
Eilersen A, Sneppen K. SARS-CoV-2 superspreading in cities vs the countryside. APMIS: acta pathologica, microbiologica. et Immunol Scand. 2021;129(7):401–7. https://doi.org/10.1111/apm.13120. PubMed PMID: 33622024; PubMed Central PMCID: PMCPMC8013868. eng.
Ssentongo P, Ssentongo AE, Voleti N, et al. SARS-CoV-2 vaccine effectiveness against Infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2022;22(1):439. https://doi.org/10.1186/s12879-022-07418-y. PubMed PMID: 35525973; PubMed Central PMCID: PMCPMC9077344. eng.
Fatima S, Zafar A, Afzal H, et al. COVID-19 Infection among vaccinated and unvaccinated: does it make any difference? PLoS ONE. 2022;17(7):e0270485. https://doi.org/10.1371/journal.pone.0270485. PubMed PMID: 35839210; PubMed Central PMCID: PMCPMC9286242. eng.
McMenamin ME, Nealon J, Lin Y et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. The Lancet Infectious diseases. 2022;22(10):1435–1443. doi: 10.1016/s1473-3099(22)00345-0. PubMed PMID: 35850128; PubMed Central PMCID: PMCPMC9286709 GlaxoSmithKline, Moderna, Pfizer, Roche, and Sanofi Pasteur. JN was previously employed by and owns shares in Sanofi. All other authors declare no competing interests. eng.
Sifuentes Giraldo WA, García Villanueva MJ, Boteanu AL et al. New targets in systemic lupus (part 2/2). Reumatologia clinica. 2012 Sep-Oct;8(5):263-9. https://doi.org/10.1016/j.reuma.2012.01.013. PubMed PMID: 22483664; eng.
Woodruff MC, Ramonell RP, Nguyen DC, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020;21(12):1506–16. https://doi.org/10.1038/s41590-020-00814-z. PubMed PMID: 33028979; PubMed Central PMCID: PMCPMC7739702. eng.
Kim CW, Oh JE, Lee HK. Single cell transcriptomic re-analysis of Immune cells in Bronchoalveolar Lavage Fluids reveals the correlation of B cell characteristics and Disease Severity of patients with SARS-CoV-2 Infection. Immune Netw. 2021;21(1):e10. https://doi.org/10.4110/in.2021.21.e10. PubMed PMID: 33728103; PubMed Central PMCID: PMCPMC7937513. eng.
Allard-Chamard H, Kaneko N, Bertocchi A, et al. Extrafollicular IgD(-)CD27(-)CXCR5(-)CD11c(-) DN3 B cells infiltrate inflamed tissues in autoimmune fibrosis and in severe COVID-19. Cell Rep. 2023;42(6):112630. https://doi.org/10.1016/j.celrep.2023.112630. PubMed PMID: 37300833; PubMed Central PMCID: PMCPMC10227203. eng.
Szelinski F, Stefanski AL, Schrezenmeier E, et al. Plasmablast-like phenotype among Antigen-experienced CXCR5-CD19(low) B cells in systemic Lupus Erythematosus. Arthritis & Rheumatology (Hoboken NJ). 2022;74(9):1556–68. https://doi.org/10.1002/art.42157. PubMed PMID: 35507291; eng.
Chung MKY, Gong L, Kwong DL, et al. Functions of double-negative B cells in autoimmune Diseases, Infections, and cancers. EMBO Mol Med. 2023;15(9):e17341. https://doi.org/10.15252/emmm.202217341. PubMed PMID: 37272217; PubMed Central PMCID: PMCPMC10493577. eng.
Zhang F, Zheng J, Li Y, et al. Phase 3, long-term, open-label extension period of safety and efficacy of belimumab in patients with systemic Lupus Erythematosus in China, for up to 6 years. RMD open. 2022;8(1). https://doi.org/10.1136/rmdopen-2021-001669. PubMed PMID: 35428697; PubMed Central PMCID: PMCPMC9014060. eng.
Doria A, Bass D, Schwarting A, et al. A 6-month open-label extension study of the safety and efficacy of subcutaneous belimumab in patients with systemic Lupus Erythematosus. Lupus. 2018;27(9):1489–98. doi: 10.1177/0961203318777634. PubMed PMID: 29807477; PubMed Central PMCID: PMCPMC6066857. eng.
Struemper H, Thapar M, Roth D, Population Pharmacokinetic, and Pharmacodynamic Analysis of Belimumab Administered Subcutaneously in Healthy Volunteers and Patients with Systemic Lupus Erythematosus. Clinical pharmacokinetics. 2018;57(6):717–728. doi: 10.1007/s40262-017-0586-5. PubMed PMID: 28887801; PubMed Central PMCID: PMCPMC5973992 Struemper is a former employee ofowns stock in GlaxoSmithKline. David Roth is an employee ofowns stock in GlaxoSmithKline. Mita Thapar has no conflicts of interest directly relevant to the contents of this study. ETHICS APPROVAL: All procedures performed in studies involving human participants were in accordance with the ethical standards of the investigational review board or human subjects committeewith the 1964 Helsinki Declarationits later amendments or comparable ethical standards. CONSENT TO PARTICIPATE: Informed consent was obtained from all individual participants included in the study. eng.
Levy RA, Gonzalez-Rivera T, Khamashta M, et al. 10 years of belimumab experience: what have we learnt? Lupus. 2021;30(11):1705–21. 10.1177/09612033211028653. PubMed PMID: 34238087; PubMed Central PMCID: PMCPMC8564244. eng.
Tanaka Y, Bae SC, Bass D, et al. Long-term open-label continuation study of the safety and efficacy of belimumab for up to 7 years in patients with systemic Lupus Erythematosus from Japan and South Korea. RMD open. 2021;7(2). https://doi.org/10.1136/rmdopen-2021-001629. PubMed PMID: 34215703; PubMed Central PMCID: PMCPMC8256836. eng.
Ugarte-Gil MF, Alarcón GS, Izadi Z, et al. Characteristics associated with poor COVID-19 outcomes in individuals with systemic Lupus Erythematosus: data from the COVID-19 Global Rheumatology Alliance. Ann Rheum Dis. 2022;81(7):970–8. https://doi.org/10.1136/annrheumdis-2021-221636. PubMed PMID: 35172961; PubMed Central PMCID: PMCPMC8882632. eng.
Mehta P, Gasparyan AY, Zimba O, et al. Systemic Lupus Erythematosus in the light of the COVID-19 pandemic: Infection, vaccination, and impact on Disease management. Clin Rheumatol. 2022;41(9):2893–910. https://doi.org/10.1007/s10067-022-06227-7. PubMed PMID: 35639259; PubMed Central PMCID: PMCPMC9152659. eng.
Alunno A, Najm A, Machado PM et al. 2021 update of the EULAR points to consider on the use of immunomodulatory therapies in COVID-19. Annals of the rheumatic Diseases. 2022;81(1):34–40. https://doi.org/10.1136/annrheumdis-2021-221366. PubMed PMID: 34620584; PubMed Central PMCID: PMCPMC8507408. eng.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (82001728), 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant Number: ZYGD18015, ZYJC18003) and Medical Research Program of Sichuan Province (S21016).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CYT and YL jointly proposed the study topic and jointly designed the study protocol with YHL. YLW and TW designed the questionnaire and screened the list of potential participants. DYH completed the relevant ethical review. JHW, WHZ, XJJ, and CQY followed up and verified the cohort patients by telephone according to the list and data. ZHL completed the data collation and induction. YLW, XPL, and LC performed the data analysis and interpretation of the results. YLW and YHL wrote the manuscript, and CYT, YL and MH revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This retrospective study received ethical approval from the Biomedical Ethics Committee of West China Hospital, Sichuan University. The approval number assigned to this study is 2023 (277). This study was a retrospective observational study, without any intervention in the process of patient care, and all data did not contain information that could identify the patient’s identity and would not harm the patient’s privacy, so informed consent was not applicable. The study did not involve animal studies, and no ethical approval was needed.
Ethics declarations
This study was approved by the ethical committee of West China Hospital of Sichuan University (No. 277 in 2023) and complied with the Declaration of Helsinki.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Li, Y., Wu, T. et al. COVID-19 in Systemic Lupus Erythematosus patients treated with belimumab: a retrospective clinical study. Immunol Res (2023). https://doi.org/10.1007/s12026-023-09449-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12026-023-09449-2