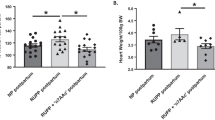
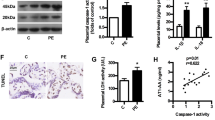
Abstract
Preeclamptic women are reported to have inadequate plasma volume expansion coupled with a suppressed secretion of aldosterone; however, the specific mechanism of preeclampsia remains unclear. We demonstrated that the presence of long-term angiotensin II type 1 receptor autoantibody (AT1-AA) reduces aldosterone production by triggering a Ca2+ overload in H295R cells. AT1-AA was discovered in preeclamptic women and reported to activate AT1R, and consequently elevate intracellular Ca2+. We found that AT1-AA significantly prolonged the time of intracellular Ca2+ elevation. Besides promoting aldosterone production as a second messenger, Ca2+ overload shows a cytotoxic effect. Our data reveals that long-term presence of AT1-AA triggered a Ca2+ overload and consequent impairment of aldosterone production, which could be prevented by a PKC inhibitor, Gö 6983, or a calcium channel inhibitor, nifedipine. These findings have clinical significance because AT1R blockers are not recommended for treatment of preeclampsia due to their potential harm to the fetus. Our findings also emphasize a potential clinical benefit of immunoadsorption or neutralization of AT1-AA in preeclamptic women.




Similar content being viewed by others
References
Lindheimer MD, Umans JG. Explaining and predicting preeclampsia. N Engl J Med. 2006;355(10):1056–8. https://doi.org/10.1056/NEJMe068161.
de Haas S, Ghossein-Doha C, van Kuijk SM, van Drongelen J, Spaanderman ME. Physiological adaptation of maternal plasma volume during pregnancy: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49(2):177–87. https://doi.org/10.1002/uog.17360.
Gallery ED, Hunyor SN, Gyory AZ. Plasma volume contraction: a significant factor in both pregnancy-associated hypertension (pre-eclampsia) and chronic hypertension in pregnancy. Q J Med. 1979;48(192):593–602.
Escher G, Mohaupt M. Role of aldosterone availability in preeclampsia. Mol Asp Med. 2007;28(2):245–54. https://doi.org/10.1016/j.mam.2007.03.002.
Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–52. https://doi.org/10.1172/JCI4106.
Siddiqui AH, Irani RA, Zhang W, Wang W, Blackwell SC, Kellems RE, et al. Angiotensin receptor agonistic autoantibody-mediated soluble fms-like tyrosine kinase-1 induction contributes to impaired adrenal vasculature and decreased aldosterone production in preeclampsia. Hypertension. 2013;61(2):472–9. https://doi.org/10.1161/HYPERTENSIONAHA.111.00157.
Yang J, Li L, Shang JY, Cai L, Song L, Zhang SL, et al. Angiotensin II type 1 receptor autoantibody as a novel regulator of aldosterone independent of preeclampsia. J Hypertens. 2015;33(5):1046–56. https://doi.org/10.1097/HJH.0000000000000521.
Wei M, Zhao C, Zhang S, Wang L, Liu H, Ma X. Preparation and biological activity of the monoclonal antibody against the second extracellular loop of the angiotensin II type 1 receptor. J Immunol Res. 2016;2016:1858252. https://doi.org/10.1155/2016/1858252.
Yu L, Yang J, Wang X, Jiang B, Sun Y, Ji Y. Antioxidant and antitumor activities of Capparis spinosa L. and the related mechanisms. Oncol Rep. 2017;37(1):357–67. https://doi.org/10.3892/or.2016.5249.
Irani RA, Xia Y. Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin Nephrol. 2011;31(1):47–58. https://doi.org/10.1016/j.semnephrol.2010.10.005.
Gennari-Moser C, Khankin EV, Schuller S, Escher G, Frey BM, Portmann CB, et al. Regulation of placental growth by aldosterone and cortisol. Endocrinology. 2011;152(1):263–71. https://doi.org/10.1210/en.2010-0525.
de Groot CJ, Taylor RN. New insights into the etiology of pre-eclampsia. Ann Med. 1993;25(3):243–9.
Bussen SS, Sutterlin MW, Steck T. Plasma renin activity and aldosterone serum concentration are decreased in severe preeclampsia but not in the HELLP-syndrome. Acta Obstet Gynecol Scand. 1998;77(6):609–13.
Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, et al. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993;133(4):1555–61. https://doi.org/10.1210/endo.133.4.8404594.
Yang X, Wang F, Chang H, Zhang S, Yang L, Wang X, et al. Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. J Hypertens. 2008;26:1629–35.
Abadir PM, Jain A, Powell LJ, Xue QL, Tian J, Hamilton RG, et al. Discovery and validation of agonistic angiotensin receptor autoantibodies as biomarkers of adverse outcomes. Circulation. 2017;135:449–59.
Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Mol Cell Endocrinol. 2004;217(1–2):67–74. https://doi.org/10.1016/j.mce.2003.10.011.
Schafer C, Shahin V, Albermann L, Schillers H, Hug MJ, Oberleithner H. Intracellular calcium: a prerequisite for aldosterone action. J Membr Biol. 2003;196(3):157–62. https://doi.org/10.1007/s00239-003-0634-7.
Williams TA, Monticone S, Crudo V, Warth R, Veglio F, Mulatero P. Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension. 2012;59(4):833–9. https://doi.org/10.1161/HYPERTENSIONAHA.111.188532.
Zhang S, Zheng R, Yang L, Zhang X, Zuo L, Yang X, et al. Angiotensin type 1 receptor autoantibody from preeclamptic patients induces human fetoplacental vasoconstriction. J Cell Physiol. 2013;228(1):142–8. https://doi.org/10.1002/jcp.24113.
Aguilera G, Catt KJ. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology. 1986;118(1):112–8. https://doi.org/10.1210/endo-118-1-112.
Ueki N, Takeda S, Koya D, Kanasaki K. The relevance of the renin-angiotensin system in the development of drugs to combat preeclampsia. Int J Endocrinol. 2015;2015:572713. https://doi.org/10.1155/2015/572713.
Funding
This work was supported by the grants from the Major Research plan of the National Natural Science Foundation of China (Grant No. 91539205) to Huirong Liu, the National Natural Science Foundation of China (Grant No.81471478) to Xiaoli Yang, and the Natural Science Foundation of Shanxi Province, China (Grant No.201601D011093) to Feng Wang.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The protocol was approved by the Ethics Committee of Capital Medical University (Beijing, China).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 773 kb)
Rights and permissions
About this article
Cite this article
Lei, J., Zhang, S., Wang, P. et al. Long-term presence of angiotensin II type 1 receptor autoantibody reduces aldosterone production by triggering Ca2+ overload in H295R cells. Immunol Res 66, 44–51 (2018). https://doi.org/10.1007/s12026-017-8963-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-017-8963-6