Abstract
Our laboratory is interested in how immunogenicity may be modulated in vivo in order to better design more effective immunotherapeutics against cancer. Our main approach is to use a facultative intracellular bacterium, Listeria monocytogenes, which has the unusual ability to live and grow in the cytoplasm of the cell and is thus an excellent vector for targeting passenger antigens to the major histocompatibility complex (MHC) class I pathway of antigen processing with the generation of authentic CTL epitopes. We have used this approach to target tumor antigens expressed on breast, melanoma and cervical cancer. We are also exploring the role of Listerial virulence factors in potentiating adaptive immune responses by activating innate immunity. Specifically, we are using these proteins as adjuvants for B cell lymphomas.
Similar content being viewed by others
Introduction
Our laboratory has long been interested in the properties of proteins that render them immunogenic and how such immunogenicity may be modulated in vivo. In the case of the immunoglobulin receptor on B cells both the antigenic site on the protein antigen and the binding site of the immunoglobulin are topographic surfaces. However, in the case of the T cell receptor the antigenic region of the protein is a peptide derived by cellular processing which is expressed on the surface of an antigen presenting cell associated with a molecule of the major histocompatibility complex (MHC). The cellular compartment in which the T cell epitope is generated determines whether it emerges at the cell surface bound to MHC class I or II molecules and, therefore, what type of T cell response is elicited. There have been enormous advances made in the last few years in our understanding of the molecular and cellular machinery that governs the presentation of antigens to the immune system. In our laboratory, we are attempting to apply this knowledge to the development of strategies of immune regulation for cancer. Our work serves two purposes. The first is that we believe that we can learn much about the mechanisms of immunity in testing current theories by manipulating immunopathological states. The second is to heed Albert Sabin’s maxim “To yield to every whim of curiosity, and to allow our passion for inquiry to be restrained by nothing but the limits of our ability shows an eagerness of mind not unbecoming to scholarship. But it is wisdom that has the merit of selecting from among the innumerable problems which present themselves, those whose solution is important to mankind.” [1].
In recent years, we have focused our efforts largely on attempting to enhance the immunogenicity of tumor antigens to provide more effective immunotherapeutics for cancer. Our main approach is to use a facultative intracellular bacterium, Listeria monocytogenes, which has the unusual ability to live and grow in the cytoplasm of the cell. We have shown that recombinant forms of this organism which have been transformed to express foreign antigens are excellent vectors for targeting foreign antigens to the MHC class I pathway of antigen processing with the generation of authentic CTL epitopes. In the past we have successfully used this unusual vector to target viral antigens from influenza, HIV, and SIV and tumor associated antigens [2]. However, vaccine approaches to target tumor antigens have to overcome additional problems to those presented by vaccines against infectious disease. Antigens expressed by cancer cells are often strongly homologous to self-antigens and induce tolerance in the host. Tumors have also evolved a number of suppressive mechanisms to evade immune detection. We have found that L. monocytogenes is unusually effective in overcoming these hurdles and shows great promise as a tumor immunotherapeutic.
Listeria monocytogenes as a vaccine vector
We have long believed that the type of immunity induced by the facultative intracellular parasite Listeria monocytogenes could be ideal for boosting the immune response to foreign antigens [2]. It has been shown that L. monocytogenes enters the host cell and is taken up in a phagosome. However, unlike most other intracellular bacteria [3], L. monocytogenes escapes from the phagosome into the cytoplasm of the cell by disrupting the phagosomal membrane, primarily through the action of listeriolysin O. The bacteria replicate in the cytoplasm, and then move to the periphery of the cell where they form pseudopod-like structures that are recognized and internalized by adjacent cells where the cycle is repeated [4]. The unusual ability of L. monocytogenes to escape phagolysosomal restriction and live in the cytoplasm explains why this bacterium is particularly effective as a vector for targeting the class I restricted pathway of antigen processing. The localization of bacteria in the lysosomal compartment after invasion ensures that antigens expressed by this bacterium enter the MHC class II pathway for antigen processing since the majority of bacteria are killed and digested in this compartment in vivo [5]. Finally, the ability of the bacterium to spread from cell to cell without entering the extra-cellular matrix explains its inadequacy in inducing an antibody response [6].
Early studies showed that CD8+ T cells are the major effector subset in controlling and clearing the listerial murine infection [7, 8]. Hemolytic activity of the bacteria is required for T-cell induction in vivo and presentation to CD8+ T cells in vitro [9, 10]. The role of CD4+ T cells appears to be less critical for the control of L. monocytogenes infections in the murine model. Depletion of CD4+ lymphocytes during adoptive transfer has a much less pronounced effect than depletion of CD8+ lymphocytes [7]. Depletion of CD4+ cells during primary L. monocytogenes infection or in L. monocytogenes immune mice had only a marginal effect in controlling L. monocytogenes challenge, although delayed-type hypersensitivity and granuloma formation were severely diminished in mice treated with anti-CD4 antibody [8]. The strong DTH response to L. monocytogenes can now be directly related to the innate immune response to this bacterium that takes place early in infection [11]. Phagocytosis of Listeria monocytogenes by a resident macrophage results in the secretion of IL-1, IL-12, and TNF-α and its activation to a state, which is competent to destroy the invading parasite by nitric oxide (NO) production. IL-1 secreted by resident macrophages activates neutrophils and helps to maintain the activated state of macrophages, which have increased MHC expression. IL-12 and TNF-α act on NK cells stimulating them to secrete IFN-γ. This facilitates the expansion of Th0 cells which under the influence of IL-12 and IFN-γ differentiate to the Th1 phenotype that secrete IL-2, TNF-α, and IFN-γ. Later in infection, IL-10 may be secreted by macrophages, which can downregulate the activation of Th0–Th1. These stages in innate immunity are consistent with the ability of L. monocytogenes to promote cell-mediated over humoral immunity and verify that the in vivo CD4+ T cell response is also restricted to a Th1 phenotype as is the case with other intracellular pathogens [6, 12].
Listeria monocytogenes as a cancer immunotherapeutic
From studies performed in the past two decades, there has been an emerging consensus that T cells are the critical mediators of an effective anti-tumor response [13]. Unlike antibody targets, T cell epitopes may be cytoplasmically located and their epitopes consist of linear amino acid stretches. A large number of tumor antigens as well as epitopes recognized by tumor-specific T cells have now been identified [14]. Consequently, current cancer vaccination strategies are primarily directed toward raising strong tumor-specific T cell responses and introducing these identified T cell antigens to the immune system in such a way as to prime an immune response that will be sufficient to eliminate tumor metastases and residual tumor mass [13]. A serious problem, however, in mounting an immune response to antigens expressed by tumor cells is that they are often poorly immunogenic because of their strong homology to self-proteins [15]. In addition, they will have been initially presented to the immune system in the context of tumor cells that are, for the most part, poor antigen presenting cells likely to induce tolerance to the antigen rather than an active T cell response.
The ability of Listeria monocytogenes to stimulate strong innate and cell-mediated immunity suggests that this bacterium may be an ideal vaccine vector to introduce poorly immunogenic tumor-specific antigens to the MHC class I and class II antigen presentation pathways in professional antigen presenting cells. To test the efficacy of L. monocytogenes as a cancer vaccine, we first used a model tumor system, which utilized influenza nucleoprotein as a model tumor antigen [16–19]. A recombinant Listeria monocytogenes vaccine strain (Lm-NP) that expresses nucleoprotein (NP) from influenza strain A/PR8/34 was used. Lm-NP had previously been shown to present the Kd restricted NP epitope in vitro and induce NP-specific CTL in vivo [20]. To provide transplantable tumors expressing NP as a model tumor antigen, we used highly tumorigenic, class I+/II− tumor cells transduced with the NP gene. We found that the therapeutic potential of this vaccine vector to limit tumor growth [16–18] was impressive. Regression of macroscopic tumors could be demonstrated for all tumor types and was dependent on antigen-specific CD4+ and CD8+ T cells.
The frequent failure of the immune system to respond to tumor antigens and the subsequent outgrowth of transformed cells in cancer has been attributed to inadequacies of tumor cells in presenting antigens [13, 14] and to tolerance mechanisms acting upon tumor-specific T cells [15]. This deficiency can be remedied by transfecting the tumor cells with cytokine genes [21] or with costimulatory ligands [22] that either activate or bypass the requirement for T cell and help in mounting a protective tumor-specific CD8+ CTL response. Accordingly, the Th1 type cytokine profile induced by Listeria monocytogenes that we have described may play an important role in the efficacy of this vaccine vector. Indeed, we have demonstrated the presence of mRNA from a wide array of Th1 type cytokines in regressing tumors explanted from vaccinated animals and shown that depletion of the Th1 type cytokines IL-12, IFN-γ, and TNF-α can abrogate the ability of L. monocytogenes to protect against tumor challenge in the NP model system [19]. The induction by Th1 cytokines of cell surface molecules important in T cell activation is a considerable advantage in an anti-cancer vaccine. Thus, Listeria monocytogenes has been shown to be an ideal vehicle not only for presentation of tumor antigens to tumor-specific T cells but also to provide the correct milieu to enhance the efficacy of these effector cells.
Within the last few years, the importance of IFN-γ has been recognized as an essential component of successful immunotherapy of HPV-transformed tumors. Loss of responsiveness to IFN-γ has been considered a possible mechanism responsible for the ability of many human tumors to avoid immune recognition and clearance. Earlier work in the lab demonstrated the dependence of Listeria vaccine efficacy on IFN-γ in order to induce tumor regression of an HPV-16 immortalized tumor [23]. The effects of IFN-γ signaling were studied using tumor cells that expressed a “dominant-negative” mutated form of the IFN-γ receptor, in a series of tumor regression studies as well as in vitro functional assays. Results from these studies showed that vaccination with Listeria vaccines was most efficacious in the presence of appropriate IFN-γ signaling and associated MHC I upregulation by target tumor cells. Vaccination with a recombinant Listeria expressing HPV-16 E7 induced complete regression in the majority of the mice bearing wild type tumors when compared to mice bearing tumors unresponsive to IFN-γ Moreover, lymphocyte infiltration into the tumor was also dependent on intact IFN-γ signaling with a marked retention of potential effector cells within the capsule of the unresponsive tumor. Current research in the laboratory is focused on understanding the mechanisms underlying IFN-γ mediated tumor T cell infiltration and effector function.
Listeria monocytogenes as a vector for human tumor antigens
In the past five years, we have focused our attention on targeting clinically relevant tumor antigens. We first chose a tumor antigen that is present in the majority of cervical cancer cells. Human papillomaviruses (HPV) comprise a diverse family of double-stranded DNA viruses that infect epithelial tissues. More than 90% of cervical carcinomas have detectable levels of HPV DNA, most commonly of the HPV 16 genotype [24]. Expression of the HPV 16 early gene products, E6 and E7, are sufficient to immortalize human and rodent cells [24]. These proteins are constitutively expressed by HPV associated tumors and are necessary for maintenance of the transformed phenotype. They are, therefore, ideal candidates for target antigens in tumor immunotherapy [24].
To test the anti-tumor effectiveness of the E7-specific immune responses induced by E7 secreting Listeria strains we used a lung epithelial cell immortalized by HPV-16 E6 and E7 and transformed by pVEJB expressing activated human c-Ha-ras called TC-1 [25]. It is an aggressive tumor, syngeneic with the C57Bl/6 mouse.
We constructed two Listeria strains that secrete E7, but they were generated with two different expression systems. Lm-E7 possesses a single copy of the E7 gene and the expression and secretion of the recombinant protein is driven by the hly promoter and signal sequence. The second strain, Lm-LLO-E7, expresses and secretes a fusion protein consisting of a truncated listeriolysin-O (LLO) joined at the C-terminus to E7 [26]. Although both Listeria monocytogenes recombinants secrete the E7 tumor antigen they induce radically different anti-tumor responses. Lm-LLO-E7 treatment effectively cures the majority of tumor bearing mice. In vivo antibody depletion studies demonstrated that the anti-tumor response requires CD4+ and CD8+ T cells as well as IFN-γ. Alternatively, Lm-E7 treatment of tumor bearing mice had little impact on the growth of the tumor.
An interesting question arises from these studies as to why fusing the E7 antigen to LLO enhances the anti-tumor efficacy of the vaccine. This effect is not due to the ability of listeriolysin to dissolve endosomal membranes because the fusion protein is constructed with a truncated version of LLO that has lost the domain responsible for the lytic activity. We hypothesized that the enhanced immunity conferred by fusing an antigen to LLO is at least partly due to the presence of a Proline (P), Glutamic acid (E), Serine (S), Threonine (T) (PEST) sequence at the amino terminus of LLO [27]. To test this hypothesis, TC-1 tumor bearing C57BL/6 mice were vaccinated with Listeria recombinants expressing HPV-16 E7 fused to LLO and containing or lacking the LLO PEST sequence [28]. These tumor regression studies showed that vaccines lacking the PEST sequence were less effective at inducing tumor regression with complete regression of established tumors occurring in the majority of animals given PEST sequence containing vaccines. Vaccines containing PEST sequences also induced greater percentages of splenic and tumor infiltrating, antigen-specific CD8+ lymphocytes. The results of these experiments suggested a link between tumor regression and the ability of PEST containing vaccines to induce specific CTLs that were capable of entering the tumor microenvironment.
PEST sequences in eukaryotic proteins are not only rich in the amino acids proline, glutamic acid, serine, and threonine, they are generally flanked by clusters containing several positively charged residues. These sequences serve to target the protein to the ubiquitin-proteosome pathway for degradation; thus, the presence of this sequence in LLO may target the protein for rapid destruction by host cell proteolytic machinery [27]. In order to verify that the efficacy of the fusion protein is independent of the vector used to deliver the antigen, we constructed a recombinant vaccinia virus that carried the gene for the fusion protein LLO-E7. We then demonstrated that it was a much more potent cancer immunotherapeutic for HPV immortalized tumors than an isogenic strain of vaccinia that carries the gene for E7 protein alone or one that expresses the E7 protein fused to a lysosomal associated membrane protein signal sequence to better target the antigen to the MHC class II pathway for antigen processing and presentation [29]. Interestingly, another of Listeria’s virulence factors, ActA, also contains PEST sequences. ActA is required for the assembly of the actin filaments that characterize the propulsion and intercellular infection of Listeria [4]. We thus engineered a recombinant strain of Listeria monocytogenes to express and secrete HPV-16 E7 as a fusion protein with ActA. Studies assessing the ability of this vaccine strain of Listeria revealed complete regression of established, E7-expressing TC-1 tumors in C57BL/6 mice while inducing E7-specific cytotoxic T-lymphocyte activity [30].
In addition to enhanced proteasomal degradation and presentation on MHC I, we hypothesized that differences in the efficacy of the two vaccines were linked, in part, to the ability of each of the vaccine strains to render otherwise immature professional antigen presenting cells such as dendritic cells (DCs) into effective, mature APCs [31]. In vitro comparisons of Lm-E7, Lm-LLO-E7, and wild-type Listeria monocytogenes revealed enhanced upregulation by Lm-LLO-E7 of MHC II as well as CD40, CD80, CD86, B7-HI, and B7-DC on bone marrow derived DCs. Cytokine production was also monitored for each vaccine along with wild-type L. monocytogenes and all were capable of inducing high levels of TNF-α and IL-12 but only Lm-LLO-E7 stimulated high levels of IL-2 protein. The increased levels of IL-2 in Lm-LLO-E7 suggest that LLO-fused antigens may have a greater antitumor effect due to enhanced activation of naïve T cells. Activation of naïve T cells was investigated for both vaccines and the evidence demonstrated that Lm-LLO-E7 pulsed DCs increase T cell proliferation while Lm-E7 pulsed DCs show little effect. Finally, Lm-LLO-E7 pulsed DCs when injected along with TC-1 tumor cells lead to 75% of mice remaining tumor-free, while Lm-E7 pulsed DCs only lead to 37.5% of mice being tumor-free. The summation of these results demonstrates that Lm-LLO-E7 enhances activation of immature DCs leading to a more robust antitumor response in comparison to the Lm-E7 vaccine, which may explain the differences observed in previous experiments.
While these studies suggested that enhanced presentation of the antigen and increased dendritic cell maturation are likely mechanisms to describe the adjuvant properties afforded by antigen fusion with LLO, we investigated further the necessity of this fusion. In order to separate the non-specific effects of Lm infection from the adjuvant effect of LLO for these studies, we employed the use of a eukaryotic expression vector to produce a series of DNA vaccines [31]. The DNA vaccines expressed either E7 alone (pcDNA3.1-E7), LLO alone (pcDNA3.1-LLO), LLO fused to E7 (pcDNA3.1-LLOE7), or LLO and E7 expressed as a bicistronic message (pcDNA3.1-LLO-IRES-E7). The vaccines were administered i.m. along with plasmids expressing MIP-1α and GM-CSF to test for anti-tumor efficacy against an established tumor expressing E7 (TC-1). Once again, fusion of LLO to E7 produced a significant anti-tumor effect in comparison to just E7 alone as demonstrated by pcDNA3.1-LLOE7 vaccination leading to 63% of mice being tumor-free in comparison to no mice in the pcDNA3.1-E7 vaccination. Interestingly, the vaccinations with LLO expressed along with E7 but not fused (LLO + E7mix and LLO-IRES-E7) also demonstrated anti-tumor efficacy with 38% of mice being tumor free. We also found that fusion of LLO to E7 was not required to enhance the E7-specific CD4+ cell response but fusion was necessary to induce an E7-specific CD8 + cell response. The E7-specific CD4+ response was further characterized and found not to increase E7-specific IgG suggesting that the CD4+ T cells induced by LLOE7 are CD4+ Th1 cells. In summary, this evidence suggests that LLO alone can act to increase MHC II presentation of antigens likely through upregulation of co-stimulatory molecules and MHC. However, fusion of LLO to the antigen is required to stimulate a strong antigen-specific CD8+ T cell response and to achieve the greatest anti-tumor efficacy suggesting that enhanced MHC I presentation plays an important role in the adjuvant properties of LLO.
The ability of LLO within the context of a Listeria-based vaccine to stimulate the maturation of dendritic cells suggests that LLO may be capable of activating an inflammatory cytokine cascade much like a Pathogen-Associated Molecular Pattern (PAMP). Recent publications have suggested that LLO can stimulate the transcription and release of inflammatory cytokines, but it is unclear if this event is independent of its cytolytic activity [32–34]. We hypothesize that a detoxified form of LLO, such as the form used in our vaccine strategy, can also activate an inflammatory cascade by interacting with Pattern Recognition Receptors (PRRs) and leading to inflammatory cytokine release and dendritic cell maturation. We are investigating LLO PAMP activity by utilizing purified protein in an effort to avoid non-specific vector consequences. Purified antigen alone, fused with LLO, or mixed with LLO are being assessed for the ability to stimulate inflammatory cytokine transcription and release in immature bone marrow dendritic cells. The ability of LLO to mature bone marrow dendritic cells will also be determined, followed up with anti-tumor efficacy against established tumors in vivo. If it is found that LLO is acting as a PAMP, we will then continue to dissect its activation pathway by identifying its specific PRR. The information obtained from this study could assist us in rational design of a more active detoxified version of LLO to be utilized in our vaccine strategies.
Listeriolysin O as a vaccine adjuvant for non-Hodgkin’s lymphoma
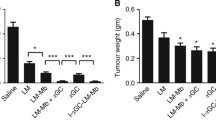
We have built on our findings that LLO has adjuvant properties to explore its ability to enhance the immunogenicity of the B cell idiotype expressed by lymphomas [35]. Currently the most successful, experimental immunotherapeutic approach to non-Hodgkin’s lymphoma is a cancer “vaccine” comprising the idiotype of the patient’s own B cell lymphoma coupled to keyhole limpet hemocyanin (KLH). Non-Hodgkin’s B cell lymphomas usually express a clonotypic B cell receptor (BCR). The VH and VL genes of this unique receptor can be cloned and expressed as a fusion protein linked by a single flexible polypeptide chain [36]. This construct is called scFv. There is a great deal of evidence that a strong anti-idiotypic humoral response to the scFv is the anti-tumor effector mechanism in this approach. The choice of KLH as the carrier protein to provide T cell help for this response and to help break tolerance to this self-tumor associated antigen is largely historical [37]. KLH has been used for decades to study hapten carrier relationships. However, there has not been a serious search for better protein carriers that could perhaps enhance the immunity to the idiotype. We are taking advantage of our recent finding, described above, that fusing an antigen to a truncated form of LLO from Listeria monocytogenes improves the immunogenicity of the antigen regardless of the delivery vector [26, 27, 29, 31]. We hypothesize that this bacterial protein, and other Listerial proteins, which also contain PEST sequences, may prove to be a more potent carrier to break tolerance to B cell idiotypes. To test this hypothesis, we used a mouse model for B cell lymphoma that expresses a monoclonal B cell receptor (BCR). The 38C13 tumor is a carcinogen-induced B cell lymphoma that expresses an IgM kappa BCR. It was originally derived from the C3H mouse and has been used for decades as a mouse model for human lymphoma [38]. Indeed, almost all current idiotype based immunotherapeutics that have moved into the clinic were originally tested in this model. Our results demonstrated that an LLO-fused anti-idiotype (Id-LLO) protein vaccine matches or exceeds the efficacy of a KLH-fused anti-idiotype (Id-KLH) protein vaccine in enhancing systemic immunity and tumor protection against the aggressive lymphoma cell line, 38C13 [35]. The Id-LLO vaccine also provided for a powerful Th1 response as evidenced by increased IgG2a anti-Id antibodies after one immunization that persisted and increased frequency of CD4+ T cells secreting IFN-γ in the draining lymph nodes. Alternatively, Id-KLH required two immunizations to reach the same antibody titer. Additionally, Id-LLO provided better protection against the BCR loss variant 38C12-V2 after initial challenges with 38C12 suggesting that it increased epitope spreading to a greater degree than Id-KLH. This study provides evidence that incorporation of LLO, rather than KLH, into anti-Id vaccines may be more efficacious in the treatment of B-cell lymphoma [35].
Listeria monocytogenes as a vector to overcome T cell tolerance
A major challenge faced by the growing field of immunotherapeutics targeting various cancers is the ability of such treatments to overcome host immune tolerance mechanisms acting upon tumor-specific T cells [15]. The extent to which Listeria-based vaccines were able to break central and peripheral tolerance had not been generally studied and compelled us to further analyze the role of specific CD4+ T cell subsets in the induction or suppression of tumor immunity [26]. The depletion of CD4+ T cells greatly improves the effectiveness of the Lm-E7 treatment with approximately 25% of treated mice undergoing complete regression of established TC-1 tumors. The transfer of CD4+ T cells from mice immunized with Lm-E7 can abrogate the anti-TC1 immune response induced by Lm-LLO-E7-treated recipient mice. This finding led us to address the role of regulatory T cells, which are known to be important for the maintenance of self-tolerance. The depletion of Tregs, which are known to suppress both proliferation and IFN-γ production by CD8+ T cells [39], can result in the induction of autoimmunity. CD4+, CD25+ T cells have also been demonstrated to aid tumor growth by suppressing anti-tumor immune responses [40]. A series of experiments were conducted in order to examine the prevalence and the activities of regulatory T cells induced in animals vaccinated with the vaccine constructs. We found increased levels of splenic and tumor infiltrating CD4+, CD25+ T cells in mice vaccinated with Lm-E7 compared to mice vaccinated with Lm-LLO-E7. In vitro analyses of isolated CD4+, CD25+ cells showed that these cells were capable of suppressing effector T cell proliferation and, in the case of tumor infiltrating CD4+ T cells, producing suppressive cytokines such as TGFβ and IL-10 [41]. A similar link between the presence of LLO in an anti-tumor vaccine and the suppression of Treg function was recently reported in an Escherichia coli based vaccine study [42]. Current studies in our laboratory are focused on investigating potential links between our previously mentioned work in the areas of effective antigen presentation and the suppression of otherwise unfavorable Treg responses.
While peripheral tolerance mechanisms may be addressed in part by additional studies of the differential effects of antigen processing, central tolerance remains a major concern for any immunotherapeutic treatments. To that end, recent work in the laboratory focused on the development of suitable models to test the dynamics, the efficacy, and the ability of previously generated Listeria-based vaccines to break central tolerance [43]. Specifically, a new mouse model was constructed in which we examined immune tolerance to the tissue-specific tumor associated antigens E6 and E7. Characteristics of the E6/E7 transgenic mice included enlarged thyroids as well as E7 expression in mTECs isolated from the thymus of E6/E7 transgenic mice. Tumor regression studies were conducted using TC-1 tumor bearing transgenic C57BL/6 mice in which E7 proteins were expressed as tissue-specific self-antigens. Listeria E7 vaccines were shown to be less effective at inducing complete tumor regression in the transgenic mice compared to their wild type counterparts. Furthermore, there was a reduction in the frequency of E7-specific CTLs derived from vaccinated, tumor bearing transgenic mice, which displayed diminished cytolytic activity and were judged to possess a lower functional avidity relative to wild type mice. However, despite a reduction in the efficacy of T cells induced by Listeria-based vaccines in transgenic mice, these cells retain some capacity to infiltrate and control tumor growth. Removal of Tregs in these studies failed to increase vaccine efficacy in transgenic mice suggesting that this peripheral tolerance mechanism was not responsible for CTL suppression, further implicating central tolerance mechanisms. These experiments led us to conclude that our Listeria-based vaccines overcome central tolerance by expanding E7 specific, low avidity CD8+ T cells that are not deleted during thymic development and are capable of eliminating solid tumors.
Listeria monocytogenes as a vector for breast tumor antigens
We have initiated two new tumor immunotherapy programs that target breast cancer. In one, we are using Listeria monocytogenes vector as the vector for Her-2/neu, a member of the epidermal growth factor receptor family that is present in a constitutively active form on up to 60% of human breast tumors [44]. Because Her-2/neu is very large (1,260 residues) and is also membrane bound, we constructed five different Listeria recombinants each of which expresses a single fragment of Her-2/neu. The amino acid sequences contained in each Her-2/neu fragment were 20-326 in Lm-LLO-EC1, 303-501 in Lm-LLO-EC2, 479–655 in Lm-LLO-EC3, 690–1081 in Lm-LLO-IC1, and 1020–1260 in Lm-LLO-IC2.
The models we used to test these vaccines were a transplantable NT-2 model in the FVB/N mouse [45] and a tolerance model using a transgenic FVB/N mouse that overexpresses the rat Her-2/neu gene under the control of the MMTV promoter. These mice spontaneously develop tumors between 4 and 9 months of age [46, 47]. All five vaccines were equally able to induce regression of the implantable, Her-2/neu expressing, tumor line (NT-2) in the wild type FVB/N mouse [48]. Previous studies in the FVB/N mouse described only one CTL epitope [45], which is located in the EC2 fragment of our vaccines. In this study, Ercolini et al., [45] used irradiated whole 3T3 cells transduced with Her-2/neu and GM-CSF as a vaccine, in addition to a vaccinia vaccine containing full length Her-2/neu, to generate T cell clones. They then used these T cell clones and overlapping peptides in CTL analyses to determine the sequence of the EC2 epitope as PDSLRDLSVF. All of the T cell clones generated, responded to this single peptide, and for this reason, was determined to be the immunodominant epitope for Her-2/neu in the FVB/N mouse [45]. In contrast, we used Listeria monocytogenes based vaccines containing fragments of Her-2/neu. We did not use T cell clones, but instead we used a polyclonal primary T cell pool in our CTL analyses to identify CD8+ T cell epitopes. By this approach, we have found not one, but at least thirteen different CTL epitopes [48–51]. Our method of vaccination, therefore, appears to reveal cryptic epitopes that were previously overlooked, implying that the vaccination strategy can determine the immunodominance of epitopes.
We also tested our five Lm-LLO-Her-2/neu vaccines for their ability to eradicate NT-2 in the transgenic mouse [51]. We found that they were all equally capable of slowing or halting tumor growth despite the fact that the CD8+ cytotoxic T cells isolated from immunized mice were of lower avidity than those from wild type mice [51]. We also tested the ability of the Lm-LLO-Her-2/neu vaccines to delay the appearance of spontaneous tumors in the transgenic Her-2/neu mouse [49]. Interestingly, the vaccines differed in their ability in this regard. When we tracked the tumors that eventually grew out, we found the tumors that emerged had mutated residues within CTL epitopes of the Her-2/neu molecule. These mutations resided in the exact regions that were targeted by the Listeria vaccines, which suggested that the rate of generation of escape mutants was a significant factor in the efficacy of each vaccine. Thus, mutations in the Her-2/neu regions allowed for escape mutants from immunotherapy to form, leading to the outgrowth of spontaneous tumors in the transgenic Her-2/neu mouse model [49].
A longer delay in the onset of tumors after immunotherapy occurred with the vaccine that targeted the kinase domain, which is contained in the fragment IC1. We verified that the mutations in this domain occurred within novel CD8+ T cell epitopes and that the mutation of these residues abrogated cytotoxic T cell lymphocyte responses to these epitopes [49]. The long delay in the onset of tumors after immunotherapy targeting the kinase domain may be because this region of Her-2/neu cannot undergo extensive mutations without impairing the ability of the molecule to signal cell growth. Thus, tumors targeted by Lm-LLO-IC1 may grow out after a delay due to a decrease in signaling and a subsequent decrease in the proliferative capacity of the tumors cells.
Recently, we have embarked on a new strategy in targeting breast tumors, rather than direct killing of tumor cells we are targeting molecules important in tumor angiogenesis. One such molecule, the High Molecular Weight Melanoma Antigen (HMW-MAA), also known as Melanoma Chondroitin Sulfate Proteoglycan (MCSP), is overexpressed in 90% of all surgically removed benign nevi and melanoma lesions [52]. HMW-MAA can also be found expressed by basal cell carcinoma, tumors of the neural crest origin, and in some forms of childhood leukemias. In addition, HMW-MAA has also been found at high levels on both activated pericytes and pericytes in tumor angiogenic vasculature [53, 54]. We had originally constructed a Listeria-based vaccine that expressed three different fragments of HMW-MAA molecule: HMW-MAA-A (AA 360–554), HMW-MAA-B (AA 701–1130), and HMW-MAA-C (AA 2160–2258), all three fragments were expressed but only the transmembrane portion of the HMW-MAA molecule (HMW-MAA-C) showed any efficacy in inducing regression of established B16 and RENCA transplantable tumors. Taking advantage of the fact that HMW-MAA is found on pericytes, and knowing that pericytes are required for the proper functioning of vessels and communication with the underlying endothelium, we hypothesized that immunizing against HMW-MAA on pericytes could possibly cause disruption of the tumor vasculature and possibly cause tumor death. This was tested using a transplantable breast tumor system using the Her-2/neu expressing cell line NT-2. Immunization of mice with established breast tumors using the Lm-LLO-HMW-MAA-C construct could, in fact, induce regression of growing NT-2 tumors, cause epitope spreading to various regions of the endogenous tumor antigen Her-2/neu, and reduce the tumor’s microvascular density as assessed by immunohistochemistry [55]. In addition, we were able to reduce pericyte coverage in regressed tumors, all without harm to normal angiogenesis as evidence by proper wound healing and pregnancy studies.
These studies, taken together, provide evidence that Listeria can overcome tolerance to self-antigen and expand autoreactive T cells normally too low in number and avidity to drive anti-tumor responses. The also provide an optimistic view for the use of Listeria in the treatment of cancer.
Concluding Remarks
It is obvious that the outcome of the immune response to a protein antigen, whether foreign or related to self, is governed by a complex series of in vivo events. These include the antigen processing pathway accessible to the antigen, the level of co-stimulatory ligands on the antigen presenting cell, the cytokine milieu in which the immune response develops and the possible induction of immuno-regulatory cells. In this brief review, we have touched on the use of the live recombinant vector, Listeria monocytogenes, to manipulate these parameters. In pre-clinical mouse tumor models, Listeria has shown unusual potency at eradicating established tumors. It still remains to be seen if this promise will translate to clinical efficacy. However, clinical trials are currently underway for the use of Lm-LLO-E7 as a cancer immunotherapeutic for cervical cancer (http://www.advaxis.com/lc.htm).
References
Sabin A. Presidential Address to the Infectious Disease Society of America. 1983.
Paterson Y. Rational approaches to immune regulation. Immunol Res. 2003;27:451–62. (Cancro M, Monroe J, editors).
Paterson Y. The relationship between bacterial life-styles and the immune responsiveness to bacterially delivered antigens. In: Paterson Y, editor. Intracellular bacteria as live recombinant vaccine vectors Immunology cell biology and genetics. New York, NY: Wiley; 1999. p. 1–24.
Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608.
Portnoy DA, Schreiber RD, Connelly P, Tilney LG. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989;170:2141–6.
Kaufmann SHE. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63.
Czuprynski CJ, Brown JF. Dual regulation of anti-bacterial resistance and inflammatory neutrophil and macrophage accumulation by L3T4+ and Lyt 2+ Listeria immune T cells. Immunology. 1987;60:287–93.
Mielke ME, Ehlers S, Hahn H. T-cell subsets in delayed-type hypersensitivity, protection and granuloma formation in primary and secondary Listeria infection in mice: Superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988;56:1920–5.
Brunt LM, Portnoy DA, Unanue ER. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–6.
Berche P, Gaillard J, Sansonetti PJ. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–71.
Weiskirch L, Paterson Y. The use of Listeria monocytogenes recombinants as vaccine vectors in infectious and neoplastic disease. In: Paterson Y, editor. Chapter 7 in Intracellular bacteria as live recombinant vaccine vectors: Immunology, cell biology and genetics. New York, NY: Wiley; 1999. p. 223–59.
Vijh S, Pamer EG. The cell biology and immune response to Listeria monocytogenes, an intracellular pathogen. In: Paterson Y, editor. Chapter 3 in Intracellular bacteria as live recombinant vaccine vectors: Immunology, cell biology and genetics. New York, NY: Wiley; 1999.
Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15.
Jones LA, Salgaller ML. Immunologic approaches to antigen discovery for cancer vaccines. Expert Opin Investig Drugs. 2000;9:481–90.
Pardoll DM. Inducing autoimmune disease to treat cancer. Proc Natl Acad Sci USA. 1999;96:5340–2.
Pan Z-K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour challenge and causes regression of established tumours. Nat Med. 1995;1:471–7.
Pan Z-K, Ikonomidis G, Pardoll D, Paterson Y. Regression of established tumors in mice mediated by the oral administration of a recombinant Listeria monocytogenes vaccine. Cancer Res. 1995;55:4776–9.
Pan Z-K, Weiskirch LM, Paterson Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 1999;59:5264–9.
Weiskirch LM, Pan Z-K, Paterson Y. The tumor recall response of anti-tumor immunity primed by a live, recombinant L. monocytogenes vaccine is comprised of multiple effector mechanisms. Clin Immunol. 2001;98:346–57.
Ikonomidis G, Paterson Y, Kos F, Portnoy D. Delivery of a viral antigen to the class I processing and presentation pathway by L. monocytogenes. J Exp Med. 1994;180:2209–18.
Jaffee EM, Pardoll DM. Considerations for the clinical development of cytokine gene-transduced tumor cell vaccines. Methods. 1997;12:143–53.
Antonia SJ. B7-1 gene-modified tumor cell vaccines. Curr Opin Mol Ther. 1999;1:50–6.
Dominiecki ME, Beatty GL, Pan Z-K, Neeson P, Paterson Y. Tumor sensitivity to IFNγ is required for successful antigen-specific immunotherapy of a transplantable mouse tumor model for HPV-transformed tumors. Cancer Immunol Immunother. 2005;54:477–88.
Tindle RW. Human papilloma virus vaccines for cervical cancer. Curr Opin Immunol. 1996;8:643–50.
Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6.
Gunn GR, Zubair A, Peters CH, Pan Z-K, Wu T-C, Paterson Y. Two L. monocytogenes vaccine vectors that express different molecular forms of HPV-16 E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–9.
Gunn GR, Peters C, Paterson Y. Listeriolysin––a useful cytolysin. Trends in Microbiol. 2001;9:161–2.
Sewell DA, Shahabi V, Gunn GR III, Pan Z-K, Dominiecki ME, Paterson Y. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor associated antigen human papillomavirus-16 E7. Cancer Res. 2004;64:8821–5.
Lamikanra A, Pan Z-K, Isaacs S, Wu T-C, Paterson Y. The ability to induce the regression of established HPV-16 immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8+ T cell responses that home to the tumor site. J Virol. 2001;75:9654–64.
Sewell DA, Douven D, Pan Z-K, Rodriguez A, Paterson Y. Regression of HPV-positive tumors treated with a new Listeria monocytogenes vaccine. Arch Otolaryngol Head Neck Surg. 2004;130:92–7.
Peng X, Treml J, Paterson Y. Adjuvant properties of listeriolysin O protein in a DNA vaccination strategy. Cancer Immunol Immunother. 2007;56:797–806.
Noor S, Goldfine H, Tucker DE, Suram S, Lenz LL, Akira S, et al. Activation of cytosolic phospholipase A2alpha in resident peritoneal macrophages by Listeria monocytogenes involves listeriolysin O and TLR2. J Biol Chem. 2008;283:4744–55.
Nishibori T, Xiong H, Kawamura I, Arakawa M, Mitsuyama M. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect Immun. 1996;64:3188–95.
Kohda C, Kawamura I, Baba H, et al. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect Immun. 2002;70:1334–41.
Neeson P, Pan ZK, Paterson Y. Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma. Cancer Immunol Immunother. 2008;57:493–505.
Hawkins RE, Zhu D, Ovecka M, Winter G, Hamblin TJ, Long A, et al. Idiotypic vaccination against human B-cell lymphoma. Rescue of variable region gene sequences from biopsy material for assembly as single-chain Fv personal vaccines. Blood. 1994;83:3279–88.
Bergman Y, Haimovich J. Characterization of a carcinogen-induced murine B lymphocyte cell line of C3H/eB origin. Eur J Immunol. 1977;7:413–17.
Kaminski MS, Kitamura K, Maloney DG, Levy R. Idiotype vaccination against murine B cell lymphoma. Inhibition of tumor immunity by free idiotype protein. J Immunol. 1987;138:1289–96.
Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40.
Anthony PA, Restifo NP. Do CD4+ CD25+ immunoregulatory T cells hinder tumor immunotherapy? J Immunother. 2002;25:202–6.
Hussain SF, Paterson Y. CD4+ CD 25+ Regulatory T cells that secrete TGFβ and IL-10 are preferentially induced by a vaccine vector. J Immunother. 2004;27(5):339–46.
Nitcheu-Tefit J, Dai M-S, Critchley-Thorne RJ, Ramirez-Jimenez F, Xu M, Conchon S, et al. Listeriolysin O expressed in a bacterial vaccine suppresses CD4+ CD25 high regulatory T cell function in vivo. J Immunol. 2007;179:1532–41.
Souders NC, Sewell DA, Pan Z-K, Hussain SF, Rodriguez A, Wallecha A, et al. Listeria based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 2007;7:1–12.
Bernhard H, Salazar L, Schiffman K, Smorlesi A, Schmidt B, Knutson KL, et al. Vaccination against the HER-2/neu oncogenic protein. Endocr Relat Cancer. 2002;9:33–44.
Ercolini AM, et al. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol. 2003;170:4273–80.
Muller WJ. Expression of activated oncogenes in the murine mammary gland: transgenic models for human breast cancer. Cancer Metastasis Rev. 1991;10:217–27.
Guy CT, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–82.
Singh R. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–73.
Singh R, Paterson Y. Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res. 2007;67:1887–92.
Singh R, Paterson Y. Vaccination strategy determines the emergence and dominance of CD8 + T-cell epitopes in a FVB/N rat HER-2/neu mouse model of breast cancer. Cancer Res. 2006;66:7748–57.
Singh R, Paterson Y. In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines. Cancer Immunol Immunother. 2007;56:927–38.
Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–96.
Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–11.
Von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312(5):623–9.
Maciag P, Seavey M, Pan Z, Ferrone S, Paterson Y. Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res. 2008;68:8066–75.
Acknowledgments
We thank all the current and past members of the Paterson laboratory for their contributions to these studies and, in particular Dr. Zhen-Kun Pan, for her unswerving support and industry for the past 18 years. Yvonne Paterson wishes to disclose that she has a financial interest in Advaxis, Inc., a vaccine and therapeutic company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of Listeria or listerial products as vaccines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Laurence M. Wood, Patrick D. Guirnalda, Matthew M. Seavey are made an equal contribution to this work.
Rights and permissions
About this article
Cite this article
Wood, L.M., Guirnalda, P.D., Seavey, M.M. et al. Cancer immunotherapy using Listeria monocytogenes and listerial virulence factors. Immunol Res 42, 233–245 (2008). https://doi.org/10.1007/s12026-008-8087-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-008-8087-0