Abstract
Objectives
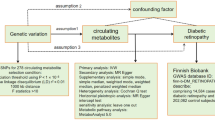
There is ample that metabolic dysregulation is involved in Graves’ disease (GD) and Graves’ ophthalmopathy (GO). Recent studies have identified numerous metabolites associated with GD and GO. However, the causal impact of metabolites on GD and GO remains to be investigated.
Methods
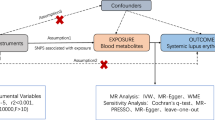
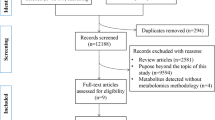
This two-sample Mendelian randomization (MR) analysis investigated the causal relationships between 486 blood metabolites and GD and GO. Sensitivity analysis was also performed to examine heterogeneity and pleiotropy.
Results
MR analysis showed that 9 and 13 metabolites were associated with GD and GO, respectively, each meeting the nominal significance criteria (inverse variance weighted, p < 0.05). Additionally, four metabolic pathways were identified for each condition using network-based MetaboAnalyst 5.0.
Conclusions
The metabolites and pathways discovered in this study could serve as circulating metabolic biomarkers for clinical screening and prevention of GD and GO. They can be also used for further studies on the mechanisms and drug targets in GD and GO.


Similar content being viewed by others
References
S.Y. Lee et al. Hyperthyroidism: A Review. JAMA 330(15), 1472–1483 (2023)
T.J. Smith et al. Graves’ Disease. N. Engl. J. Med. 375(16), 1552–1565 (2016)
T. Milo et al. Autoimmune thyroid diseases as a cost of physiological autoimmune surveillance. Trends Immunol. 44(5), 365–371 (2023)
J. Cao et al. The risk factors for Graves’ ophthalmopathy. Graefes Arch. Clin. Exp. Ophthalmol. 260(4), 1043–1054 (2022)
L. Bartalena et al. Epidemiology, Natural History, Risk Factors, and Prevention of Graves’ Orbitopathy. Front Endocrinol. 11, 615993 (2020)
R. Ma et al. Insights Into Ferroptosis: Targeting Glycolysis to Treat Graves’ Orbitopathy. J. Clin. Endocrinol. Metab. 107(7), 1994–2003 (2022)
X. Chen et al. Influence of 4-week or 12-week glucocorticoid treatment on metabolic changes in patients with active moderate-to-severe thyroid-associated ophthalmopathy. Clin. Transl. Sci. 14(5), 1734–1746 (2021)
H.O. Ueland et al. Systemic Activation of the Kynurenine Pathway in Graves Disease With and Without Ophthalmopathy. J. Clin. Endocrinol. Metab. 108(6), 1290–1297 (2023)
S.K. Byeon et al. Lipidomic differentiation of Graves’ ophthalmopathy in plasma and urine from Graves’ disease patients. Anal. Bioanal. Chem. 410(27), 7121–7133 (2018)
D.Y. Ji et al. Comparative assessment of Graves’ disease and main extrathyroidal manifestation, Graves’ ophthalmopathy, by non-targeted metabolite profiling of blood and orbital tissue. Sci. Rep. 8(1), 9262 (2018)
X. Zhang et al. Exploring blood metabolites and thyroid disorders: a bidirectional mendelian randomization study. Front Endocrinol. 14, 1270336 (2023)
B.L. Pierce et al. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 178(7), 1177–1184 (2013)
J. Bowden et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 40(4), 304–314 (2016)
S. Burgess et al. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32(5), 377–389 (2017)
A.F. Schmidt et al. Mendelian randomization with Egger pleiotropy correction and weakly informative Bayesian priors. Int J. Epidemiol. 47(4), 1217–1228 (2018)
C. Xue et al. Tryptophan metabolism in health and disease. Cell Metab. 35(8), 1304–1326 (2023)
Y. Chang et al. Tryptophan 2,3-dioxygenase 2 plays a key role in regulating the activation of fibroblast-like synoviocytes in autoimmune arthritis. Br. J. Pharm. 179(12), 3024–3042 (2022)
K. Åkesson et al. Kynurenine pathway is altered in patients with SLE and associated with severe fatigue. Lupus Sci. Med. 5(1), e000254 (2018)
Y. Park et al. Kynurenine pathway can be a potential biomarker of fatigue in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 41(12), 2363–2370 (2023)
T. Bögl et al. Plasma Metabolomic Profiling Reveals Four Possibly Disrupted Mechanisms in Systemic Sclerosis. Biomedicines 10(3), 607 (2022)
S.M.T. Isık et al. Relationship of tryptophan metabolites with the type and severity of multiple sclerosis. Mult. Scler. Relat. Disord. 77, 104898 (2023)
A. Krupa et al. The Kynurenine Pathway-New Linkage between Innate and Adaptive Immunity in Autoimmune Endocrinopathies. Int J. Mol. Sci. 22(18), 9879 (2021)
S.H. Chu et al. Circulating plasma metabolites and risk of rheumatoid arthritis in the Nurses’ Health Study. Rheumatology 59(11), 3369–3379 (2020)
C. Chen et al. Metabolomic profiling reveals amino acid and carnitine alterations as metabolic signatures in psoriasis. Theranostics 11(2), 754–767 (2021)
X. Cheng et al. Long-Chain Acylcarnitines Induce Senescence of Invariant Natural Killer T Cells in Hepatocellular Carcinoma. Cancer Res. 83(4), 582–594 (2023)
U. Wenzel et al. Increased mitochondrial palmitoylcarnitine/carnitine countertransport by flavone causes oxidative stress and apoptosis in colon cancer cells. Cell Mol. Life Sci. 62(24), 3100–3105 (2005)
M.C. Mutomba et al. Regulation of the activity of caspases by L-carnitine and palmitoylcarnitine. FEBS Lett. 478(1-2), 19–25 (2000)
B. Kisiel et al. Polymorphism of the oestrogen receptor beta gene (ESR2) is associated with susceptibility to Graves’ disease. Clin. Endocrinol. 68(3), 429–434 (2008)
C.W. Cheng et al. Possible interplay between estrogen and the BAFF may modify thyroid activity in Graves’ disease. Sci. Rep. 11(1), 21350 (2021)
A.M. FitzPatrick, Is Estrogen a Missing Culprit in Thyroid Eye Disease? Sex Steroid Hormone Homeostasis Is Key to Other Fibrogenic Autoimmune Diseases - Why Not This One? Front Immunol. 13, 898138 (2022)
H.B. Burch et al. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves’ ophthalmopathy. Exp. Eye Res 65(2), 311–316 (1997)
T. Mano et al. Changes in free radical scavengers and lipid peroxide in thyroid glands of various thyroid disorders. Horm. Metab. Res 29(7), 351–354 (1997)
R. Du et al. Metabolic features of orbital adipose tissue in patients with thyroid eye disease. Front. Endocrinol. 14, 1151757 (2023)
J. Zhou et al. Detection and Correlation Analysis of Serum Uric Acid in Patients with Thyroid-Associated Ophthalmopathy. Comput. Math. Methods Med. 2022, 8406834 (2022)
N.F. Marques et al. Guanosine Protects Striatal Slices Against 6-OHDA-Induced Oxidative Damage, Mitochondrial Dysfunction, and ATP Depletion. Neurotox. Res. 35(2), 475–483 (2019)
B. Bellaver et al. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic. Signal 11(4), 571–580 (2015)
L.E. Bettio et al. Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress. Pharm. Biochem. Behav. 127, 7–14 (2014)
T.Y. Hou et al. The Role of Oxidative Stress and Therapeutic Potential of Antioxidants in Graves’ Ophthalmopathy. Biomedicines 9(12), 1871 (2021)
C.C. Tsai et al. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves’ ophthalmopathy: evidence that oxidative stress has a role in this disorder. Eye 24(9), 1520–1525 (2010)
J. Liu et al. Serum Metabolomic Patterns in Patients with Autoimmune Thyroid Disease. Endocr. Pr. 26(1), 82–96 (2020)
T.J. Smith, Insulin-Like Growth Factor Pathway and the Thyroid. Front Endocrinol. 12, 653627 (2021)
P. Castrogiovanni et al. Effects of high-tryptophan diet on pre- and postnatal development in rats: a morphological study. Eur. J. Nutr. 53(1), 297–308 (2014)
F. Liang et al. Tobacco carcinogen induces tryptophan metabolism and immune suppression via induction of indoleamine 2,3-dioxygenase 1. Signal Transduct. Target Ther. 7(1), 311 (2022)
L. Zhu et al. Advancing metabolic networks and mapping updated urinary metabolic fingerprints after exposure to typical carcinogenic heterocyclic aromatic amines. Environ. Pollut. 319, 120936 (2023)
R.D. Michalek et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186(6), 3299–3303 (2011)
Z. Wu et al. Purine metabolism-related genes and immunization in thyroid eye disease were validated using bioinformatics and machine learning. Sci. Rep. 13(1), 18391 (2023)
Acknowledgements
The authors’ gratitude is extended to the GWAS consortia for openly providing the summary statistics. The authors also wish to convey appreciation to the researchers and participants involved in these studies.
Funding
National Natural Science Foundation of China, Grant/Award Numbers: 82260159.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data acquisition and analysis were conducted by T.W., Y.Z., and C.W.. Manuscript revision was carried out by H.Y. and Z.L.. The final manuscript was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Zhang, Y., Wu, C. et al. Causality of blood metabolites and metabolic pathways on Graves’ disease and Graves’ ophthalmopathy: a two-sample Mendelian randomization study. Endocrine (2024). https://doi.org/10.1007/s12020-024-03761-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03761-z