Abstract
Purpose
The 8th edition of the American Joint Committee on Cancer (AJCC) staging led to a significant downstaging of well differentiated thyroid cancer patients. However, some patients who had been downstaged still experienced death. By using data from the thyroid cancer dataset of The Cancer Genome Atlas (TCGA), we aimed to find molecular features that could improve survival prediction.
Methods
TCGA data were downloaded from cBioPortal. Restaging of cases was performed according to the pathological reports.
Results
Out of 496 cases, 204 (41.1%) were downstaged, and the proportion of deaths increased in stages III and IV. TERT promoter mutations were no longer enriched in stage IV only, but significantly redistributed also in stages II and III. TERT mutation was the only alteration predictive of poor survival; however, in this series it was not independent from the AJCC staging. Five proteins (4E-BP1_pT70, Chk1_pS345, Snail, STAT5 alpha and PAI-1) were significantly associated with survival, and their use as a panel refined the risk stratification independently from the AJCC staging, with a hazard ratio for a positive result of 21.2 (95%CI 3.7–122.2, P = 0.0006).
Conclusions
In the TCGA series, the proportion of deaths is in line with the expected survival of the latest AJCC staging, with a neat separation of risk among stages. Nevertheless, the use of protein expression can be useful in refining the stratification. Finally, after the restaging, a considerable number of tumors with TERT mutations will be allocated in lower stages; hence, dedicated studies should define the prognostic usefulness of these mutations in low-stage diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The American Joint Committee on Cancer (AJCC) tumor-node-metastasis staging is the most effective and used stratification system designed to predict survival of patients. The 8th edition of the AJCC staging for thyroid cancer was released at the end of 2016 but is effective from the beginning of 2018. With respect to the 7th edition, the main changes for well differentiated thyroid cancer (WDTC) were: (i) the increase of the age cutoff from 45 to 55 years, (ii) the redefinition of T3 disease, which now does not encompass minimal extrathyroidal extension (ETE) detected only on histological examination, and (iii) the downgrading of positive lymph nodes to stage II for older patients [1, 2]. The consequent massive downstaging was intentional, due to the overall excellent survival of patients with WDTC, especially the youngest ones. The downstaging affected both stages III and IV that comprise now less than 5% of patients with WDTC. On the contrary, the amount of patients at stages I or II raised considerably [3,4,5]. Although the proportion of deaths in the lower stages decreased due to the overall increment of patients in those stages, a non-negligible number of patients who had been downstaged experienced death. Since the staging has many implications in the management of patients including treatment and follow-up, many authors have been questioning whether the current edition of the AJCC can be improved to guarantee a safer approach to a subgroup of patients [3, 6,7,8]. In particular, it was suggested that no sufficient consideration is given to the involvement of lateral lymph nodes [6, 8], T3b-T4 disease especially in patients aged 45–55 years [3, 6] and the presence of distant metastasis at diagnosis [3]. Mutations in BRAF and TERT have also been associated with disease specific mortality in papillary thyroid cancer (PTC) [9]; however, their role as independent predictor of survival is debated, and currently none of the validated risk systems includes molecular testing. By using clinical and molecular data from the thyroid cancer dataset of The Cancer Genome Atlas (TCGA) [10], we sought to identify potential features that could refine the stratification of patients independently from the AJCC staging.
Materials and methods
Acquisition of data and restaging
Mutation Annotation Format (MAF) file, clinical data, and level 3 reverse phase protein lysate microarray (rppa) data of the PTC dataset of the TCGA [10] were downloaded from cBioPortal (https://www.cbioportal.org/) [11, 12]. Clinical variables considered were histological type, age, gender, pN, pM, AJCC stage, American Thyroid Association (ATA) risk-group, distant metastasis, patient age, completeness of resection, local invasion, and tumor size (MACIS) score, patient’s vital status (as provided in the original data), persistence of disease, ETE, thyroid differentiation score (TDS), ERK score and number of nonsilent mutations. The restaging of cases was performed following the pathological report available on cBioPortal. The redistribution of cases between the 7th and the 8th edition of the AJCC staging was plotted using ggalluvial v.0.11.1 R package.
Within the MAF file, synonymous variants were ignored. Moreover, gene fusions and TERT promoter mutations that were available in separate files were manually added.
Gene mutations enrichment
The most frequent alterations were plotted using maftools v.2.2.10 Bioconductor package [13]. The enrichment of gene mutations in specific clinical features was performed by a Fisher exact text with maftools package. In details, for features with two classes the pairwise false discovery rate (FDR) was considered, whereas for features with more than two classes the groupwise FDR was taken into account. FDR below 0.05 was considered significant.
Receiver operating characteristic (ROC) analysis
For features presented as numeric variables (i.e., MACIS score, TDS, ERK score and number of nonsilent mutations) a ROC analysis was performed. Specifically, the Youden J statistic was used to select the best cutoff in discriminating patient’s vital status by the pROC v.1.15.3 R package [14]. Patients were then dichotomized according to the selected cutoffs.
As regards rppa expression data, an exploratory ROC analysis was performed following the procedures of the caret v.6.0–84 R package [15]. Proteins with an area under the curve (AUC) greater than 0.75 were selected for further analyses. Also for proteins, the best cutoff was assessed by the Youden J statistic.
Survival analyses
Survival curves were estimated by the Kaplan–Meier method, and differences among curves were tested by log-rank test using the survival v.3.1–8 R package [16]. Survival curves were plotted using survminer v.0.4.6 R package. Multivariate analysis and estimation of hazard ratio (HR) were performed by Cox regression following the procedures of survival R package. P value below 0.05 was considered significant. All analyses were performed in R environment (v.3.6.1, https://www.r-project.org/).
Results
Restaging of cases from 7th to 8th edition of the AJCC
Out of the 496 cases considered, 292 (58.9%) were confirmed in the same stage: 284 in stage I, three in stage II and five in stage IV, whereas 204 (41.1%) cases were downstaged. Details are reported in Table 1 and Fig. 1. The median follow-up was 14.3 months (IQ range 7.1–28.5 months). Considering the AJCC 7th edition staging, the proportion of deaths were 0.4% in stage I, 4.3% in stage II, 1.9% in stage III, and 14.4% in stage IV. After the restaging, the proportion of deaths were 0.7, 6.2, 25, and 40% for stages I, II, III, and IV, respectively.
Enrichment of gene mutations in specific clinical features
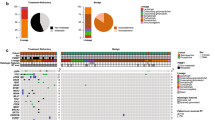
The 20 most frequent nonsilent gene alterations including fusions and TERT promoter mutations were reported in Fig. 2.
BRAF mutations were enriched in classical variant (FDR < 0.0001) and tall cell variant (TC) (FDR = 0.0007) PTC, whereas NRAS and HRAS were associated with follicular variant (FV) (FDR < 0.0001 and FDR = 0.0292 respectively). Moreover, NRAS mutations were associated with pN0 (FDR = 0.002) and ATA low-risk (FDR = 0.0077); BRAF was enriched in pN1a (FDR = 0.002), minimal ETE (FDR < 0.0001) and ATA intermediate-risk (FDR = 0.0001), whereas TERT promoter mutations were overrepresented in cases with gross ETE (FDR = 0.0018), pM1 (FDR = 0.0086) and ATA high-risk (FDR < 0.0001). In addition, TERT mutations were enriched in patients with more than 45 years (FDR = 0.006), and even strongly in those with more than 55 years (FDR < 0.0001). As regards the AJCC staging, in the 7th edition TERT was overrepresented only in stage IV (FDR < 0.0001), whereas in the 8th edition it was enriched in stages IV (FDR = 0.0061), III (FDR = 0.0049) and II (FDR = 0.0119). Finally, TERT was the only gene associated with persistence of disease (FDR = 0.0025) and death (FDR = 0.0027). Results are summarized in Table 2.
Univariate and multivariate survival analyses
The 8th edition of the AJCC staging produced a neater separation of curves than the 7th edition and a lower P value (Fig. 3). Among the other tested predictors, several were significant by univariate analysis including ATA risk stratification (P = 0.0008), ETE (P < 0.0001), age (P < 0.0001), MACIS score (P < 0.0001), TDS (P = 0.02), ERK score (P = 0.04), number of nonsilent mutations (P = 0.002) and TERT promoter mutations, both alone (P < 0.0001) or coexisting with BRAF or RAS (P < 0.0001). However, when fitting a multivariate Cox regression with the 8th edition of the AJCC, all the above-mentioned predictors were not independent.
Rppa data analysis
Since in the cases with rppa data (n = 222) the majority of adverse events were in CVPTC, and no event occurred in FVPTC and TCPTC, only CVPTC (n = 143) were considered for the following analyses. The exploratory ROC analysis revealed five proteins (4E-BP1_pT70, Chk1_pS345, Snail, STAT5 alpha and PAI-1) with an AUC greater than 0.75 in discriminating patient’s vital status. For each of these proteins, the expression value with the best Youden index was selected as cutoff. Next, patients were dichotomized according to the cutoffs, and all five proteins were significant predictors of survival. In details, poor survival was associated with a lower level of 4E-BP1_pT70, Chk1_pS345, and STAT5 alpha, and a higher level of Snail and PAI-1. Moreover, when tested in multivariate analysis with the 8th edition of the AJCC, three proteins (Chk1_p345, STAT5 alpha and PAI-1) were independent predictors of survival, whereas for 4E-BP1_pT70 and Snail Cox regression cannot be fitted because no event occurred in one of the two groups. Details were reported in Table 3. Finally, the five proteins were considered as a panel; a positive result was assigned whenever at least four out of five protein markers were above (or below) the selected cutoff. The panel was significantly associated with survival (P < 0.0001, Fig. 4), even independently from the AJCC staging, with an HR of 21.2 (95% CI 3.7–122.2, P = 0.0006).
Discussion
The TCGA study on PTC provided the most comprehensive characterization of the molecular landscape of PTC [10]; however, the available follow-up period is short, especially in the context of WDTC. After the TCGA milestone, other studies have provided a comprehensive molecular characterization of PTC, even in light of the prognostic stratification [17]. For instance, Yoo and colleagues reported that advanced WDTC often harbor secondary mutations such as TERT promoter, AKT1, PIK3CA, and EIF1AX. In addition, some WDTC have an expression profile that is different from the three molecular subtypes of PTC (i.e., BRAF-like, RAS-like, and Non-BRAF-Non-RAS, NBNR). This fourth group closely resemble to anaplastic thyroid cancer (ATC) and was consequently named ATC-like. Herein, by using TCGA clinical-pathological and molecular data, we evaluated whether one or more molecular features could help in the patients’ risk stratification, also in the light of the AJCC staging system updating.
The 8th edition of the AJCC staging system produced the downstaging of a remarkable number of cases (Fig. 1 and Table 1) with the consequent neater separation of the risk of mortality among the four stages (Fig. 3). In the TCGA series, the proportion of deaths for each stage (i.e., 0.7, 6.2, 25, and 40% for stages I, II, III, and IV respectively) was lower but in line with the 10-year projection of expected survival [1, 2].
Besides the proved usefulness of gene mutations in diagnostics [18], they can be informative also for prognosis. In the absence of secondary mutations, RAS-driven lesions are generally low risk as confirmed by their association with the follicular variant and the absence of lymph node involvement in this series. Lesions positive only for BRAF mutations were generally associated with an intermediate risk, presence of central lymph node metastasis and minimal ETE. TERT promoter mutations deserve a separate discussion. TERT mutations were in fact the only type of mutations enriched in patient with persistence of disease and an unfavorable outcome. On one hand they can be highly informative when detected preoperatively because they should be a bell tolling since the presence of a high-risk lesion is very likely [9, 19]. On the other hand, their association with older age, aggressive pathological features and advanced stage can limit their usefulness after the pathological diagnosis and staging are made. In effect, the association of TERT promoter mutations with poor survival was already proved [9, 20, 21] and herein confirmed. TERT mutations are predictive of a poor outcome both alone [9, 20, 21] and in combination with RAS or BRAF alterations [9, 22]. Nevertheless, this should not be a solved issue, also because, with the downstaging occurring, a high number of TERT mutated cases were distributed into lower stages. For this reason, a longer follow-up and dedicated studies with a higher number of mutated cases are needed to understand whether TERT promoter mutations can help stratify patients at higher risk within low-stage diseases.
The majority of the other features analysed in the present study were predictive of poor survival including a higher MACIS score, a lower TDS, ATA high risk and the total number of nonsilent mutations. However, none of them was independent when tested in multivariate analysis with the latest edition of the AJCC staging. By analysing protein expression, we found that five proteins, namely 4E-BP1_pT70, Chk1_pS345, Snail, STAT5 alpha, and PAI-1, had a good performance in discriminating patient’s vital status (Table 3). Once chosen the best cutoff, all of them efficiently dichotomized patients with very different risk of death, and at least three of them were independent from the AJCC staging. By using these proteins as a five-marker panel, with a positive result rendered whenever at least four of them are above (or below) the selected cutoff, an effective stratification of patients was obtained (Fig. 4), and, most importantly, it was independent from the AJCC staging. The protein expression analysis is routinely and widely performed, mostly by immunohistochemistry (IHC); therefore, the five-protein panel could represent an appealing strategy to refine the risk stratification of patients. The cutoffs herein obtained are optimized for expression levels from rppa analysis, therefore they must be adjusted if another technique like IHC is used. The validation of these few markers as a new tool to predict the risk of poor outcome is warranted, especially in low-stage diseases.
Finally, the low rate of adverse events and the short follow-up period of this series could represent a limitation of the present study, thus, limiting the statistical power and requiring confirmation. Although patients with WDTC are not those with a higher risk of dying of thyroid cancer, adverse events occur also in this group of patients, albeit with a lower rate as herein observed. Moreover, the distribution of cases per stage and the proportion of deaths per each stage are in line with those reported and expected; thus, our findings are based on data that could reflect a real-life series of WDTC.
In conclusion, we provided some pieces of information to be used in the stratification of patients according to the risk of death. In addition, we proposed a five-protein-based stratification strongly predictive of patients’ survival, also independently from the AJCC staging.
References
Tuttle, M., Morris, L., Haugen, B., Shah, J., Sosa, J., Rohren, E., Subramaniam, R., Hunt, J., Perrier, N.: Thyroid-differentiated and anaplastic carcinoma. In: The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. Springer International Publishing, New York (2017).
R.M. Tuttle, B. Haugen, N.D. Perrier, Updated American Joint Committee on Cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid 27, 751–756 (2017). https://doi.org/10.1089/thy.2017.0102
S. Tam, M. Boonsripitayanon, M. Amit, B.M. Fellman, Y. Li, N.L. Busaidy, M.E. Cabanillas, R. Dadu, S. Sherman, S.G. Waguespack, M.D. Williams, R.P. Goepfert, N.D. Gross, N.D. Perrier, E.M. Sturgis, M.E. Zafereo, Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and eighth editions. Thyroid. 28, 1301–1310 (2018). https://doi.org/10.1089/thy.2017.0572
L. Lamartina, G. Grani, E. Arvat, A. Nervo, M.C. Zatelli, R. Rossi, E. Puxeddu, S. Morelli, M. Torlontano, M. Massa, R. Bellantone, A. Pontecorvi, T. Montesano, L. Pagano, L. Daniele, L. Fugazzola, G. Ceresini, R. Bruno, R. Rossetto, S. Tumino, M. Centanni, D. Meringolo, M.G. Castagna, D. Salvatore, A. Nicolucci, G. Lucisano, S. Filetti, C. Durante, 8th edition of the AJCC/TNM staging system of thyroid cancer: what to expect (ITCO#2). Endocr. Relat. Cancer 25, L7–L11 (2018). https://doi.org/10.1530/ERC-17-0453
L.N. Pontius, T.O. Oyekunle, S.M. Thomas, M.T. Stang, R.P. Scheri, S.A. Roman, J.A. Sosa, Projecting survival in papillary thyroid cancer: a comparison of the seventh and eighth editions of the American Joint Commission on cancer/union for international cancer control staging systems in two contemporary national patient cohorts. Thyroid 27, 1408–1416 (2017). https://doi.org/10.1089/thy.2017.0306
P.W. Rosario, Eighth edition of AJCC staging for differentiated thyroid cancer: is stage I appropriate for T4/N1b patients aged 45–55 years? Endocrine. 56, 679–680 (2017). https://doi.org/10.1007/s12020-017-1288-3
S.A. Ghaznavi, I. Ganly, A.R. Shaha, C. English, J. Wills, R.M. Tuttle, Using the American thyroid association risk-stratification system to refine and individualize the american joint committee on cancer eighth edition disease-specific survival estimates in differentiated thyroid cancer. Thyroid. 28, 1293–1300 (2018). https://doi.org/10.1089/thy.2018.0186
H.I. Kim, K. Kim, S.Y. Park, J.-H. Choe, J.-H. Kim, J.S. Kim, Y.L. Oh, S.Y. Hahn, J.H. Shin, H.S. Ahn, S.W. Kim, T.H. Kim, J.H. Chung, Refining the eighth edition AJCC TNM classification and prognostic groups for papillary thyroid cancer with lateral nodal metastasis. Oral Oncol. 78, 80–86 (2018). https://doi.org/10.1016/j.oraloncology.2018.01.021
R. Liu, M. Xing, TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 23, R143–R155 (2016). https://doi.org/10.1530/ERC-15-0533
Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 159, 676–690 (2014). https://doi.org/10.1016/j.cell.2014.09.050
E. Cerami, J. Gao, U. Dogrusoz, B.E. Gross, S.O. Sumer, B.A. Aksoy, A. Jacobsen, C.J. Byrne, M.L. Heuer, E. Larsson, Y. Antipin, B. Reva, A.P. Goldberg, C. Sander, N. Schultz, The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012). https://doi.org/10.1158/2159-8290.CD-12-0095
J. Gao, B.A. Aksoy, U. Dogrusoz, G. Dresdner, B. Gross, S.O. Sumer, Y. Sun, A. Jacobsen, R. Sinha, E. Larsson, E. Cerami, C. Sander, N. Schultz, Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 6, pl1 (2013). https://doi.org/10.1126/scisignal.2004088
A. Mayakonda, D.-C. Lin, Y. Assenov, C. Plass, H.P. Koeffler, Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 28, 1747–1756 (2018). https://doi.org/10.1101/gr.239244.118
X. Robin, N. Turck, A. Hainard, N. Tiberti, F. Lisacek, J.-C. Sanchez, M. Müller, pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 12, 77 (2011). https://doi.org/10.1186/1471-2105-12-77
M. Kuhn, Building predictive models in R using the caret package. J. Stat. Softw. 28, 1–26 (2008). https://doi.org/10.18637/jss.v028.i05
Modeling survival data: extending the Cox model | Terry M. Therneau | Springer, https://www.springer.com/gp/book/9780387987842, Accessed 31 Jan 2020
S.-K. Yoo, Y.S. Song, E.K. Lee, J. Hwang, H.H. Kim, G. Jung, Y.A. Kim, S.-J. Kim, S.W. Cho, J.-K. Won, E.-J. Chung, J.-Y. Shin, K.E. Lee, J.-I. Kim, Y.J. Park, J.-S. Seo, Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat. Commun. 10, 2764 (2019). https://doi.org/10.1038/s41467-019-10680-5
M.N. Nikiforova, S. Mercurio, A.I. Wald, M. Barbi de Moura, K. Callenberg, L. Santana-Santos, W.E. Gooding, L. Yip, R.L. Ferris, Y.E. Nikiforov, Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 124, 1682–1690 (2018). https://doi.org/10.1002/cncr.31245
R. Liu, M. Xing, Diagnostic and prognostic TERT promoter mutations in thyroid fine-needle aspiration biopsy. Endocr. Relat. Cancer. 21, 825–830 (2014). https://doi.org/10.1530/ERC-14-0359
M. Melo, A.G. da Rocha, J. Vinagre, R. Batista, J. Peixoto, C. Tavares, R. Celestino, A. Almeida, C. Salgado, C. Eloy, P. Castro, H. Prazeres, J. Lima, T. Amaro, C. Lobo, M.J. Martins, M. Moura, B. Cavaco, V. Leite, J.M. Cameselle-Teijeiro, F. Carrilho, M. Carvalheiro, V. Máximo, M. Sobrinho-Simões, P. Soares, TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab 99, E754–E765 (2014). https://doi.org/10.1210/jc.2013-3734
C. Bournaud, F. Descotes, M. Decaussin-Petrucci, J. Berthiller, C. de la Fouchardière, A.-L. Giraudet, M. Bertholon-Gregoire, P. Robinson, J.-C. Lifante, J. Lopez, F. Borson-Chazot, TERT promoter mutations identify a high-risk group in metastasis-free advanced thyroid carcinoma. Eur. J. Cancer. 108, 41–49 (2019). https://doi.org/10.1016/j.ejca.2018.12.003
S. Moon, Y.S. Song, Y.A. Kim, J.A. Lim, S.W. Cho, J.H. Moon, S. Hahn, D.J. Park, Y.J. Park, Effects of coexistent BRAFV600E and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: a meta-analysis. Thyroid. 27, 651–660 (2017). https://doi.org/10.1089/thy.2016.0350
Acknowledgements
The results here presented are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. Open access funding provided by Università di Pisa within the CRUI-CARE Agreement.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poma, A.M., Macerola, E., Torregrossa, L. et al. Using The Cancer Genome Atlas data to refine the 8th edition of the American Joint Committee on Cancer staging for papillary thyroid carcinoma. Endocrine 72, 140–146 (2021). https://doi.org/10.1007/s12020-020-02434-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02434-x