Abstract
Purpose
Acromegaly is a systemic metabolic disease. Growth hormone (GH) have a significant impact on adipose tissue (AT). A huge reduction of serum GH after surgical treatment may cause substantial AT redistribution. The objective of this study was to illustrate the dynamic changes in distribution of facial and abdominal AT correlated with surgical treatment in patients with acromegaly.
Methods
Abdominal AT in 17 acromegaly patients (group 1) was studied longitudinally preoperatively and 1 month to 1 year postoperatively. The facial and abdominal subcutaneous AT (fSAT and aSAT) of another 17 acromegaly patients (group 2) were compared with 7 nonfunctional pituitary adenoma (NFPA) controls.
The areas of fSAT, aSAT, and visceral adipose tissue (VAT) were obtained by MRI and quantified by image analysis software, and intrahepatic lipid (IHL) was assessed by 1H magnetic resonance spectroscopy (MRS).
Results
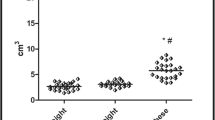
Abdominal adipose tissue (aSAT, VAT, and IHL) increased overall after surgical treatment. However, IHL first decreased and then continuously increased during the follow-up. Compared with the increased amount of aSAT, the fSAT amount decreased after surgical treatment. The inconsistency of this phenomenon did not appear in the NFPA control subjects.
Conclusion
The perioperative dynamic distribution of the facial and abdominal fat in acromegaly revealed regional differences in the intricate effect of GH on adipose tissue. Reduction of serum GH after surgical treatment of acromegaly was associated with dynamic increases of IHL, abdominal visceral, and subcutaneous fat, but a reduction of facial subcutaneous fat.




Similar content being viewed by others
References
C. Capatina, J.A. Wass, 60 years of neuroendocrinology: acromegaly. J. Endocrinol. 226(2), T141–T160 (2015)
L. Katznelson, Alterations in body composition in acromegaly. Pituitary 12, 136–142 (2009)
N. Møller, J.O. Jørgensen, Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 30(2), 152–177 (2009)
J. Dal, E.O. List, J.O. Jørgensen, D.E. Berryman, Glucose and fat metabolism in acromegaly: from mice models to patient care. Neuroendocrinology 103, 96–105 (2016)
N.C. Olarescu, J. Bollerslev, The impact of adipose tissue on insulin resistance in acromegaly. Trends Endocrinol. Metab. 27(4), 226–237 (2016)
C.M. Reyes-Vidal, H. Mojahed, W. Shen et al. Adipose tissue redistribution and ectopic lipid deposition in active acromegaly and effects of surgical treatment. J. Clin. Endocrinol. Metab. 100(8), 2946–2955 (2015)
M. Madsen, T. Krusenstjerna-Hafstrøm, L. Møller et al. Fat content in liver and skeletal muscle changes in a reciprocal manner in patients with acromegaly during combination therapy with a somatostatin analog and a ghreceptor antagonist: a randomized clinical trial. J. Clin. Endocrinol. Metab. 97(4), 1227–1235 (2012)
A. Pascale, R. Pals, V. Ratziu, An overview of nonalcoholic steatohepafitis: past, present and future directions. J. Gastrointestin. Liver. Dis. 19, 415–423 (2010)
S.M. Webb, X. Badia, Quality of life in acromegaly. Neuroendocrinology 103(1), 106–111 (2016)
D.E. Berryman, E.O. List, L. Sackmann-Sala et al. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm. IGF Res. 21(3), 113–123 (2011)
L. Katznelson, E.R. Laws Jr, S. Melmed et al. Acromegaly: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99(11), 3933–3951 (2014)
M. Maddalo, I. Zorza, S. Zubani et al. Validation of a free software for unsupervised assessment of abdominal fat in MRI. Phys. Med. 37, 24–31 (2017)
M.F. Xia, H.M. Yan, W.Y. He et al. Standardized ultrasound hepatic/renal ratio and hepatic attenuation rate to quantify liver fat content: an improvement method. Obesity 20(2), 444–452 (2012)
H.H. Hu, H.W. Kim, K.S. Nayak et al. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity 18(4), 841–847 (2010)
C.A. Schneider, W.S. Rasband, K.W. Eliceiri, NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 201(9), 671–675 (2012)
P.U. Freda, W. Shen, S.B. Heymsfield et al. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J. Clin. Endocrinol. Metab. 93(6), 2334–2343 (2008)
A. Guleria, A. Duseja, N. Kalra et al. Patients with non-alcoholic fatty liver disease (NAFLD) have an increased risk of atherosclerosis and cardiovascular disease. Trop. Gastroenterol. 34(2), 74–82 (2013)
J.H. Runge, L.P. Smits, J. Verheij et al. MR spectroscopy-derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis. Radiology 286(2), 547–556 (2018)
P. Mofrad, M.J. Contos, M. Haque et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37(6), 1286–1292 (2003)
M.A. Wagenmakers, S.H. Roerink, T.J. Maal et al. Three-dimensional facial analysis in acromegaly: a novel tool to quantify craniofacial characteristics after long-term remission. Pituitary 18(1), 126–134 (2015)
A. Tominaga, K. Arita, K. Kurisu et al. Effects of successful adenomectomy on body composition in acromegaly. Endocr. J. 45, 335–342 (1998)
A. Passaro, M.A. Miselli, J.M. Sanz et al. Gene expression regional differences in human subcutaneous adipose tissue. BMC Genomics 18(1), 202–213 (2017)
R.J. Brummer, L. Lönn, H. Kvist, et al. Adipose tissue and muscle volume determination by computed tomography in acromegaly, before and 1 year after adenomectomy. Eur J Clin Invest. 23(4), 199–205 (1993)
E.B. Geer, J. Islam, C. Buettner, Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinol. Metab. Clin. North. Am. 43(1), 75–102 (2014)
J. Weiner, M. Hankir, J.T. Heiker et al. Thyroid hormones and browning of adipose tissue. Mol. Cell. Endocrinol. 458, 156–159 (2017)
M.A. Bredella, M. Schorr, L.E. Dichtel et al. Body composition and ectopic lipid changes with biochemical control of acromegaly. J. Clin. Endocrinol. Metab. 102(11), 4218–4225 (2017)
K. Karastergiou, M.A. Bredella, M.J. Lee et al. Growth hormone receptor expression in human gluteal versus abdominal subcutaneous adipose tissue: association with body shape. Obesity 24(5), 1090–1096 (2016)
R. Hjortebjerg, D.E. Berryman, R. Comisford et al. Insulin, IGF-1, and GH receptors are altered in an adipose tissue depot-specific manner in male mice with modified GH action. Endocrinology 158(5), 1406–1418 (2017)
Acknowledgements
We thank and acknowledge all of the participants in the study. We gratefully thank TianPei for interpretation to the patients.
Funding
This study was funded by the National Key Research and Development Program of China (grant number: 2016YFC0106103).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xie, T., Ding, H., Xia, M. et al. Dynamic changes in the distribution of facial and abdominal adipose tissue correlated with surgical treatment in acromegaly. Endocrine 62, 552–559 (2018). https://doi.org/10.1007/s12020-018-1742-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1742-x