Abstract
Purpose:
MicroRNA-326 (miR-326), as a member of the microRNA (miRNA) family, which includes endogenous single-stranded, conserved, noncoding small RNAs, has been reported to play important roles in autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus. However, few studies of the role of miR-326 in autoimmune thyroiditis (AIT) have been published. Here, we explored the roles of miR-326 and the involved pathway in iodine-induced AIT.
Methods:
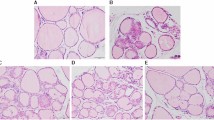
NOD.H-2h4 mice, which are a model of human AIT, were randomly divided into a normal water control group and a high-iodine group. Mice in the high-iodine group were administered 0.05% NaI (~1000 times the normal daily iodine intake), and mice in the control group received sterile water. Furthermore, we evaluated small interfering RNA (siRNA) interference in spleen mononuclear cell experiments in vitro.
Results:
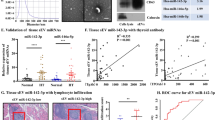
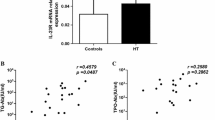
In this study, we found that Th17 cells were significantly increased with a high expression of miR-326 in an iodine-induced thyroiditis NOD.H-2h4 mouse model. In addition, the expression of Ets-1 protein, a negative regulator of Th17 differentiation, was significantly decreased. Intriguingly, our analysis showed that Ets-1 protein expression was negatively correlated with miR-326 levels in AIT mice (r = −0.814, p < 0.01). Our study indicated that miR-326 inhibited Ets-1 protein expression and promoted the differentiation of Th17 cells during the onset and development of AIT. The addition of a miR-326 inhibitor reversed Th17 cell production and Ets-1 protein expression, supporting this hypothesis.
Conclusions:
The results of our study suggest that miR-326 may target the Ets-1 protein to contribute to iodide-induced thyroiditis, providing a new theoretical basis for the use of miRNA targeting therapy for the treatment of autoimmune diseases.





Similar content being viewed by others
References
P. Caturegli, A. De Remigis, N.R. Rose, Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun. Rev. 13(4-5), 391–397 (2014).
P. Fallahi et al. The association of other autoimmune diseases in patients with autoimmune thyroiditis: review of the literature and report of a large series of patients. Autoimmun. Rev. 15(12), 1125–1128 (2016)
K. Boelaert et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am. J. Med. 123(2), 183– e1-9 (2010)
H. Braley-Mullen et al. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J. Autoimmun. 12(3), 157–165 (1999)
K. Zaletel, S. Gaberscek, Hashimoto’s thyroiditis: from genes to the disease. Curr. Genomics 12(8), 576–588 (2011)
C. Du et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 10(12), 1252–1259 (2009)
J.A. Emamaullee et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58(6), 1302–1311 (2009)
C.R. Li, E.E. Mueller, L.M. Bradley, Islet antigen-specific Th17 cells can induce TNF-alpha-dependent autoimmune diabetes. J. Immunol. 192(4), 1425–1432 (2014)
R. Kugyelka et al. Enigma of IL-17 and Th17 cells in rheumatoid arthritis and in autoimmune animal models of arthritis. Mediators Inflamm. 2016, 6145810 (2016)
A.J. Martin, L. Zhou, S.D. Miller, MicroRNA--managing the TH-17 inflammatory response. Nat. Immunol. 10(12), 1229–1231 (2009)
E. Bettelli et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090), 235–238 (2006)
C. Dong, TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 8(5), 337–348 (2008)
J. Moisan et al. Ets-1 is a negative regulator of Th17 differentiation. J. Exp. Med. 204(12), 2825–2835 (2007)
C.P. Petersen et al. Short RNAs repress translation after initiation in mammalian cells. Mol. Cell 21(4), 533–542 (2006)
R. Thermann, M.W. Hentze, Drosophila miR2 induces pseudo-polysomes and inhibits translation initiation. Nature 447(7146), 875–878 (2007)
M.D. Ballantyne, R.A. McDonald, A.H. Baker, lncRNA/MicroRNA interactions in the vasculature. Clin. Pharmacol. Ther. 99(5), 494–501 (2016)
R.M. O’Connell et al. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10(2), 111–122 (2010)
Y. Zheng, Z. Wang, Z. Zhou, miRNAs: novel regulators of autoimmunity-mediated pancreatic beta-cell destruction in type 1 diabetes. Cell. Mol. Immunol. 14(6), 488–496 (2017)
L.P. Garo, G. Murugaiyan, Contribution of MicroRNAs to autoimmune diseases. Cell Mol. Life Sci. 73(10), 2041–2051 (2016)
G. Sebastiani et al. Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes Metab. Res. Rev. 27(8), 862–866 (2011)
M.A. Honardoost et al. miR-326 and miR-26a, two potential markers for diagnosis of relapse and remission phases in patient with relapsing-remitting multiple sclerosis. Gene 544(2), 128–133 (2014)
G. Murugaiyan et al. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 187(5), 2213–2221 (2011)
X.G. Sun et al. Negative correlation between miR-326 and Ets-1 in regulatory T cells from new-onset SLE patients. Inflammation 39(2), 822–829 (2016)
P.L. Podolin et al. I-E+ nonobese diabetic mice develop insulitis and diabetes. J. Exp. Med. 178(3), 793–803 (1993)
A.O. Vladutiu, N.R. Rose, Autoimmune murine thyroiditis relation to histocompatibility (H-2) type. Science 174(4014), 1137–1139 (1971)
H. Braley-Mullen, S. Yu, NOD.H-2h4 mice: an important and underutilized animal model of autoimmune thyroiditis and Sjogren’s syndrome. Adv. Immunol. 126, 1–43 (2015)
Y. Nagayama et al. CD4+CD25+naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2h4 mice. J. Autoimmun. 29(2-3), 195–202 (2007)
I. Horie et al. T helper type 17 immune response plays an indispensable role for development of iodine-induced autoimmune thyroiditis in nonobese diabetic-H2h4 mice. Endocrinology 150(11), 5135–5142 (2009)
X. Teng et al. Experimental study on the effects of chronic iodine excess on thyroid function, structure, and autoimmunity in autoimmune-prone NOD.H-2h4 mice. Clin. Exp. Med. 9(1), 51–59 (2009)
H. Yamada et al. Circulating microRNAs in autoimmune thyroid diseases. Clin. Endocrinol. 81(2), 276–281 (2014)
J. Zhu et al. MicroRNA-142-5p contributes to Hashimoto’s thyroiditis by targeting CLDN1. J. Transl. Med. 14(1), 166 (2016)
C. Bernecker et al. MicroRNAs miR-146a1, miR-155_2, and miR-200a1 are regulated in autoimmune thyroid diseases. Thyroid 22(12), 1294–1295 (2012)
D. Li et al. Th17 cell plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin. Immunol. 149(3), 411–420 (2013)
R.X. Leng et al. The dual nature of Ets-1: focus to the pathogenesis of systemic lupus erythematosus. Autoimmun. Rev. 10(8), 439–443 (2011)
Acknowledgements
This work was supported by the Chinese National Natural Science Foundation (Grant no. 81471003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhao, N., Zou, H., Qin, J. et al. MicroRNA-326 contributes to autoimmune thyroiditis by targeting the Ets-1 protein. Endocrine 59, 120–129 (2018). https://doi.org/10.1007/s12020-017-1465-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1465-4