Abstract
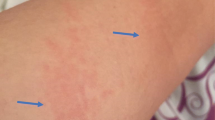
Teriparatide, or recombinant human parathyroid hormone, is approved for the treatment of osteoporosis. Possible cutaneous adverse events of teriparatide are urticaria, injection site pain, swelling, bruising, and pruritus. However, there have been no reports of widespread eczematous reactions caused by teriparatide. A 47-year-old male was recently diagnosed with osteogenesis imperfecta and prior to teeth extractions, he was subcutaneously injected with teriparatide. The patient developed multiple pruritic erythematous papules and plaques on his abdomen, around the site of the injection. A skin biopsy was done, which showed mild spongiosis and superficial perivenular lymphocytic infiltration with a few eosinophils. Drug-related eczematous changes were most likely suspected and in addition to the discontinuation of the injection, topical steroid was prescribed, in which dramatic improvements were observed. We report the eczematous hypersensitivity reaction caused by teriparatide, which is an adverse reaction that has not been reported before.


Similar content being viewed by others
References
A.H. Tashjian Jr, R.F. Gagel, Teriparatide [human PTH(1-34)]: 2.5 years of experience on the use and safety of the drug for the treatment of osteoporosis. J. Bone Miner. Res. 21(3), 354–365 (2006). doi:10.1359/jbmr.051023
M.J. Massaad, N. Ramesh, R.S. Geha, Wiskott-Aldrich syndrome: a comprehensive review. Ann. N. Y. Acad. Sci. 1285, 26–43 (2013). doi:10.1111/nyas.12049
Y. Minegishi, M. Saito, Cutaneous manifestations of Hyper IgE syndrome. Allergol. Int. 61(2), 191–196 (2012). doi:10.2332/allergolint.12-RAI-0423
J.M. White, S.E. Munn, J.E. Seet, N. Adams, M. Clement, Eczema-like plaques secondary to enoxaparin. Contact Dermatitis 54(1), 18–20 (2006). doi:10.1111/j.0105-1873.2006.00711.x
S.J. Posadas, W.J. Pichler, Delayed drug hypersensitivity reactions—new concepts. Clin. Exp. Allergy 37(7), 989–999 (2007). doi:10.1111/j.1365-2222.2007.02742.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
About this article
Cite this article
Chu, H., Kim, D.S. Eczematous dermatitis due to subcutaneous teriparatide injection. Endocrine 52, 395–396 (2016). https://doi.org/10.1007/s12020-015-0817-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0817-1