Abstract
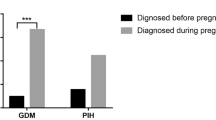
To summarize all available data on pregnancy outcome of acromegaly patients exposed to the growth hormone receptor antagonist pegvisomant (PEGV) during pregnancy as present in the Pfizer’s Global Safety Database. Pfizer’s Global Safety Database contains adverse event data obtained from the following sources: spontaneous reports, health authorities, Pfizer-sponsored post-marketing surveillance program (ACROSTUDY), customer engagement programs, and clinical studies, reported regardless of outcome. The safety database was searched up to 10th March 2014. From the 35 pregnancy cases, 27 involved maternal [mean age (range) 33.3 years (23–41) and 8 paternal (33.7 years (32–38)] PEGV exposure. Two female patients were reported with two pregnancy cases each. Fetal outcome was normal in 14 (4 paternal) of the 18 reported as live birth, while 4 cases (1 paternal) did not specify the birth outcome. At conception, PEGV mean dose (range) was 15.3 mg/d (4.3–30). In 3 cases of maternal exposure of the 18 cases reporting live birth, PEGV was continued throughout the pregnancy in a dose of 12.1 mg/d (10–15). In 5 cases (all maternal) an elective termination of the pregnancy was performed with no reported fetal abnormalities, 2 cases (maternal) reported a non-PEGV-related spontaneous abortion and in 1 maternal case an ectopic pregnancy occurred. In 9 cases (3 paternal), the fetal outcome was not reported. Three women reported gestational diabetes; one woman continued PEGV treatment during pregnancy. Although the number of reported pregnancies with exposure to PEGV is very small, the presented data reflect the largest series of data available to date and do not suggest adverse consequences of PEGV on pregnancy outcome. Nevertheless, it should be stressed that PEGV should not be used during pregnancy unless absolutely necessary.

Similar content being viewed by others
References
G.G. Brigss, R.K. Freeman, S.J. Yaffe, Drugs in Pregnancy and Lactation, 8th edn. (Lippincott Williams & Wilkins, Philadelphia, 2011)
P.J. Trainer, W.M. Drake, L. Katznelson, P.U. Freda, V. Herman-Bonert, A.J. van der Lely, E.V. Dimaraki, P.M. Stewart, K.E. Friend, M.L. Vance, G.M. Besser, J.A. Scarlett, M.O. Thorner, C. Parkinson, A. Klibanski, J.S. Powell, A.L. Barkan, M.C. Sheppard, M. Malsonado, D.R. Rose, D.R. Clemmons, G. Johannsson, B.A. Bengtsson, S. Stavrou, D.L. Kleinberg, D.M. Cook, L.S. Phillips, M. Bidlingmaier, C.J. Strasburger, S. Hackett, K. Zib, W.F. Bennett, R.J. Davis, Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N. Engl. J. Med. 342(16), 1171–1177 (2000). doi:10.1056/NEJM200004203421604
A.J. van der Lely, R.K. Hutson, P.J. Trainer, G.M. Besser, A.L. Barkan, L. Katznelson, A. Klibanski, V. Herman-Bonert, S. Melmed, M.L. Vance, P.U. Freda, P.M. Stewart, K.E. Friend, D.R. Clemmons, G. Johannsson, S. Stavrou, D.M. Cook, L.S. Phillips, C.J. Strasburger, S. Hackett, K.A. Zib, R.J. Davis, J.A. Scarlett, M.O. Thorner, Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet 358(9295), 1754–1759 (2001)
A.J. van der Lely, B.M. Biller, T. Brue, M. Buchfelder, E. Ghigo, R. Gomez, J. Hey-Hadavi, F. Lundgren, N. Rajicic, C.J. Strasburger, S.M. Webb, M. Koltowska-Haggstrom, Long-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDY. J. Clin. Endocrinol. Metab. 97(5), 1589–1597 (2012). doi:10.1210/jc.2011-2508
D. Newbern, M. Freemark, Placental hormones and the control of maternal metabolism and fetal growth. Curr. Opin. Endocrinol. Diabetes Obes. 18(6), 409–416 (2011). doi:10.1097/MED.0b013e32834c800d
P. Wiesli, C. Zwimpfer, J. Zapf, C. Schmid, Pregnancy-induced changes in insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein 3 (IGFBP-3), and acid-labile subunit (ALS) in patients with growth hormone (GH) deficiency and excess. Acta Obstet. Gynecol. Scand. 85(8), 900–905 (2006). doi:10.1080/00016340600676532
V. Cheng, C. Faiman, L. Kennedy, F. Khoury, B. Hatipoglu, R. Weil, A. Hamrahian, Pregnancy and acromegaly: a review. Pituitary 15(1), 59–63 (2012). doi:10.1007/s11102-011-0330-3
S. Cheng, L. Grasso, J.A. Martinez-Orozco, R. Al-Agha, R. Pivonello, A. Colao, S. Ezzat, Pregnancy in acromegaly: experience from two referral centers and systematic review of the literature. Clin. Endocrinol. (Oxford) 76(2), 264–271 (2012). doi:10.1111/j.1365-2265.2011.04180.x
P. Caron, S. Broussaud, J. Bertherat, F. Borson-Chazot, T. Brue, C. Cortet-Rudelli, P. Chanson, Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J. Clin. Endocrinol. Metab. 95(10), 4680–4687 (2010). doi:10.1210/jc.2009-2331
P. Maffei, G. Tamagno, G.B. Nardelli, C. Videau, C. Menegazzo, G. Milan, A. Calcagno, C. Martini, R. Vettor, J. Epelbaum, N. Sicolo, Effects of octreotide exposure during pregnancy in acromegaly. Clin. Endocrinol. (Oxford) 72(5), 668–677 (2010). doi:10.1111/j.1365-2265.2009.03706.x
P. Caron, C. Gerbeau, L. Pradayrol, Maternal-fetal transfer of octreotide [letter; comment]. N. Engl. J. Med. 333(9), 601–602 (1995)
M. Fassnacht, B. Capeller, W. Arlt, T. Steck, B. Allolio, Octreotide LAR treatment throughout pregnancy in an acromegalic woman. Clin. Endocrinol. (Oxford) 55(3), 411–415 (2001)
M.E. Molitch, Pituitary disorders during pregnancy. Endocrinol. Metab. Clin. North Am. 35(1), 99–116 (2006). doi:10.1016/j.ecl.2005.09.011
S. RiddleBrian, M. Bidlingmaier, M.P. Wajnrajch, S.A. Weinzimer, S.E. Inzucchi, Treatment of acromegaly with pegvisomant during pregnancy: maternal and fetal effects. J. Clin. Endocrinol. Metab. 92(9), 3374–3377 (2007). doi:10.1210/jc.2007-0997
Acknowledgments
ACROSTUDY is sponsored by Pfizer Inc.
Disclosure
AJvdL, received honoraria from Pfizer as member of the ACROSTUDY Strategic Advisory Board, CCH, RG, JH, A-CÅ, and PJ are permanent employees of Pfizer, MK-H was a full time employee at Pfizer Health AB when the work was done but now is an affiliate at the Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden.The authors were not paid for their contribution to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Lely, A.J., Gomez, R., Heissler, J.F. et al. Pregnancy in acromegaly patients treated with pegvisomant. Endocrine 49, 769–773 (2015). https://doi.org/10.1007/s12020-014-0508-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0508-3