Abstract
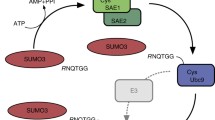
SUMOylation is a protein posttranslational modification that participates in the regulation of numerous biological processes within the cells. Small ubiquitin-like modifier (SUMO) proteins are members of the ubiquitin-like protein family and, similarly to ubiquitin, are covalently linked to a lysine residue on a target protein via a multi-enzymatic cascade. To assess the specific mechanism triggered by SUMOylation, the identification of SUMO protein substrates and of the precise acceptor site to which SUMO is bound is of critical relevance. Despite hundreds of mammalian proteins have been described as targets of SUMOylation, the identification of the precise acceptor sites still represents an important analytical challenge because of the relatively low stoichiometry in vivo and the highly dynamic nature of this modification. Moreover, mass spectrometry-based identification of SUMOylated sites is hampered by the large peptide remnant of SUMO proteins that are left on the modified lysine residue upon tryptic digestion. The present review provides a survey of the strategies that have been exploited in order to enrich, purify and identify SUMOylation substrates and acceptor sites in human cells on a large-scale format. The success of the presented strategies helped to unravel the numerous activities of this modification, as it was shown by the exemplary case of the RNA-binding protein family, whose SUMOylation is here reviewed.



Similar content being viewed by others
References
Altelaar, A. F., Munoz, J., & Heck, A. J. (2013). Next-generation proteomics: Towards an integrative view of proteome dynamics. Nature Reviews Genetics, 14, 35–48.
Babic, I., Cherry, E., & Fujita, D. J. (2006). SUMO modification of Sam68 enhances its ability to repress cyclin D1 expression and inhibits its ability to induce apoptosis. Oncogene, 25, 4955–4964.
Ban, R., Nishida, T., & Urano, T. (2011). Mitotic kinase Aurora-B is regulated by SUMO-2/3 conjugation/deconjugation during mitosis. Genes to Cells, 16, 652–669.
Becker, J., Barysch, S. V., Karaca, S., Dittner, C., Hsiao, H.-H., & Melchior, F. (2013). Detecting endogenous SUMO targets in mammalian cells and tissues. Nature Structural & Molecular Biology, 20, 525–531.
Benson, M. D., Li, Q. J., Kieckhafer, K., Dudek, D., Whorton, M. R., Sunahara, R. K., et al. (2007). SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proceedings of the National Academy of Sciences of the United States of America, 104, 1805–1810.
Blomster, H., Hietakangas, V., Wu, J., Kouvonen, P., Hautaniemi, S., & Sistonen, L. (2009). Novel proteomics strategy brings insight into the prevalence of SUMO-2 target sites. Molecular and Cellular Proteomics, 8, 1382–1390.
Blomster, H., Imanishi, S. Y., Siimes, J., Kastu, J., Morrice, N., Eriksson, J., et al. (2010). In vivo identification of sumoylation sites by a signature tag and cysteine-targeted affinity purification. Journal of Biological Chemistry, 285, 19324–19329.
Bohren, K. M., Nadkarni, V., Song, J. H., Gabbay, K. H., & Owerbach, D. (2004). A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. Journal of Biological Chemistry, 279, 27233–27238.
Bomsztyk, K., Denisenko, O., & Ostrowski, J. (2004). hnRNP K: One protein multiple processes. BioEssays, 26, 629–638.
Bruderer, R., Tatham, M. H., Plechanovova, A., Matic, I., Garg, A. K., & Hay, R. T. (2011). Purification and identification of endogenous polySUMO conjugates. EMBO Reports, 12, 142–148.
Burghes, A. H. M., & Beattie, C. E. (2009). Spinal muscular atrophy: Why do low levels of survival motor neuron protein make motor neurons sick? Nature Reviews Neuroscience, 10, 597–609.
Buschmann, T., Fuchs, S. Y., Lee, C. G., Pan, Z. Q., & Ronai, Z. (2000). SUMO-1 modification of Mdm2 prevents its self-ubiquitination and increases Mdm2 ability to ubiquitinate p53. Cell, 101, 753–762.
Chicooree, N., Griffiths, J. R., Connolly, Y., Tan, C.-T., Malliri, A., Eyers, C. E., et al. (2013). A novel approach to the analysis of SUMOylation with the independent use of trypsin and elastase digestion followed by database searching utilising consecutive residue addition to lysine. Rapid Communications in Mass Spectrometry, 27, 127–134.
Cioce, M., & Lamond, A. I. (2005). Cajal bodies: A long history of discovery. Annual Review of Cell and Developmental Biology, 21, 105–131.
Da Cruz, S., & Cleveland, D. W. (2011). Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Current Opinion in Neurobiology, 21, 904–919.
Da Silva-Ferrada, E., Lopitz-Otsoa, F., Lang, V., Rodríguez, M. S., & Matthiesen, R. (2012). Strategies to identify recognition signals and targets of SUMOylation. Biochemistry Reserch International, 2012, 875148.
Danielsen, J. R., Povlsen, L. K., Villumsen, B. H., Streicher, W., Nilsson, J., Wikström, M., et al. (2012). DNA damage-inducible SUMOylation of HERC2 promotes RNF8 binding via a novel SUMO-binding Zinc finger. Journal of Cell Biology, 197, 179–187.
Davis, B. N., Hilyard, A. C., Lagna, G., & Hata, A. (2008). SMAD proteins control DROSHA-mediated microRNA maturation. Nature, 454, 56–61.
Desterro, J. M., Keegan, L. P., Jaffray, E., Hay, R. T., Connell, M. A. O., & Carmo-fonseca, M. (2005). SUMO-1 modification alters ADAR1 editing activity. Molecular Biology of Cell, 16, 5115–5126.
Desterro, J. M., Rodriguez, M. S., & Hay, R. T. (1998). SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Molecular Cell, 2, 233–239.
Desterro, J. M., Rodriguez, M. S., Kemp, G. D., & Hay, R. T. (1999). Identification of the enzyme required for activation of the small ubiquitin-like protein SUMO-1. Journal of Biological Chemistry, 274, 10618–10624.
Desterro, J. M., Thomson, J., & Hay, R. T. (1997). Ubch9 conjugates SUMO but not ubiquitin. FEBS Letters, 417, 297–300.
Eberl, H. C., Mann, M., & Vermeulen, M. (2011). Quantitative proteomics for epigenetics. ChemBioChem, 12, 224–234.
Eichinger, C. S., & Jentsch, S. (2010). Synaptonemal complex formation and meiotic checkpoint signaling are linked to the lateral element protein Red1. Proceedings of the National Academy of Sciences of the United States of America, 107, 11370–11375.
Field, J., Nikawa, J., Broek, D., MacDonald, B., Rodgers, L., Wilson, I., et al. (1988). Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Molecular and Cellular Biology, 8, 2159–2165.
Figueroa-Romero, C., Iñiguez-Lluhí, J. A., Stadler, J., Chang, C. R., Arnoult, D., Keller, P. J., et al. (2009). SUMOylation of the mitochondrial fission protein Drp1 occurs at multiple nonconsensus sites within the B domain and is linked to its activity cycle. The FASEB Journal, 23, 3917–3927.
Finkbeiner, E., Haindl, M., & Muller, S. (2011). The SUMO system controls nucleolar partitioning of a novel mammalian ribosome biogenesis complex. EMBO Journal, 30, 1067–1078.
Flotho, A., Werner, A., Winter, T., Frank, A. S., Ehret, H., & Melchior, F. (2012). Recombinant reconstitution of sumoylation reactions in vitro. Methods in Molecular Biology, 832, 93–110.
Fok, V., Friend, K., & Steitz, J. A. (2006). Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. Journal of Cell Biology, 173, 319–325.
Galisson, F., Mahrouche, L., Courcelles, M., Bonneil, E., Meloche, S., Chelbi-Alix, M. K., & Thibault, P. (2011). A novel proteomics approach to identify SUMOylated proteins and their modification sites in human cells. Molecular & Cellular Proteomics, 10, M110.004796.
Geiss-Friedlander, R., & Melchior, F. (2007). Concepts in sumoylation: A decade on. Nature Reviews Molecular Cell Biology, 8, 947–956.
Glisovic, T., Bachorik, J. L., Yong, J., & Dreyfuss, G. (2008). RNA-binding proteins and post-transcriptional gene regulation. FEBS Letters, 582, 1977–1986.
Golebiowski, F., Matic, I., Tatham, M. H., Cole, C., Yin, Y., Nakamura, A., et al. (2009). System-wide changes to SUMO modifications in response to heat shock. Science Signaling, 26, 2.
Golebiowski, F., Tatham, M. H., Nakamura, A., & Hay, R. T. (2010). High-stringency tandem affinity purification of proteins conjugated to ubiquitin-like moieties. Nature Protocols, 5, 873–882.
Guo, D., Li, M., Zhang, Y., Yang, P., Eckenrode, S., Hopkins, D., et al. (2004). A functional variant of SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. Nature Genetics, 36, 837–841.
Haindl, M., Harasim, T., Eick, D., & Muller, S. (2008). The nucleolar SUMO-specific protease SENP3 reverses SUMO modification of nucleophosmin and is required for rRNA processing. EMBO Reports, 9, 273–279.
Hardeland, U., Steinacher, R., Jiricny, J., & Schär, P. (2002). Modification of the human thymine-DNA glycosylase by ubiquitin-like proteins facilitates enzymatic turnover. EMBO Journal, 21, 1456–1464.
Hecker, C. M., Rabiller, M., Haglund, K., Bayer, P., & Dikic, I. (2006). Specification of SUMO1- and SUMO2-interacting motifs. Journal of Biological Chemistry, 281, 16117–16127.
Hietakangas, V., Anckar, J., Blomster, H., Fujimoto, M., Palvimo, J. J., Nakai, A., et al. (2006). PDSM, a motif for phosphorylation-dependent SUMO modification. Proceedings of the National Academy of Sciences of the United States of America, 103, 45–50.
Hong, W., Resnick, R. J., Rakowski, C., Shalloway, D., Taylor, S. J., & Blobel, G. A. (2002). Physical and functional interaction between the transcriptional cofactor CBP and the KH domain protein Sam68. Molecular Cancer Research, 1, 48–55.
Hornbeck, P. V., Kornhauser, J. M., Tkachev, S., Zhang, B., Skrzypek, E., Murray, B., & Sullivan, M. (2012). PhosphoSitePlus: A comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Research, 40, D261–270.
Hsiao, H. H., Meulmeester, E., Frank, B. T. C., Melchior, F., & Urlaub, H. (2009). “ChopNSpice”, a mass spectrometric approach that allows identification of endogenous small ubiquitin-like modifier-conjugated peptides. Molecular and Cellular Proteomics, 8, 2664–2675.
Iijima, T., Wu, K., Witte, H., Hanno-Iijima, Y., Glatter, T., Richard, S., et al. (2011). SAM68 regulates neuronal activity-dependent alternative splicing of neurexin-1. Cell, 147, 1601–1614.
Johnson, E. S. (2004). Protein modification by SUMO. Annual Review of Biochemistry, 73, 355–382.
Johnson, E. S., & Blobel, G. (1997). Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. The Journal of Biological Chemistry, 272, 26799–26802.
Kim, J. S., & Raines, R. T. (1994). A misfolded but active dimer of bovine seminal ribonuclease. European Journal of Biochemistry, 224, 109–114.
Kirsh, O., Seeler, J. S., Pichler, A., Melchior, F., & Dejean, A. (2002). The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO Journal, 21, 2682–2691.
Knipscheer, P., Flotho, A., Klug, H., Olsen, J. V., Van Dijk, W. J., Fish, A., et al. (2008). Ubc9 sumoylation regulates SUMO target discrimination. Molecular Cell, 31, 371–382.
Knuesel, M., Cheung, H. T., Hamady, M., Barthel, K. K. B., & Liu, X. (2005). A method of mapping protein sumoylation sites by mass spectrometry using a modified small ubiquitin-like modifier 1 (SUMO-1) and a computational program. Molecular and Cellular Proteomics, 4, 1626–1636.
Lamoliatte, F., Bonneil, E., Durette, C., Caron-Lizotte, O., Wildemann, D., Zerweck, J., et al. (2013). Targeted identification of SUMOylation sites in human proteins using affinity enrichment and paralog-specific reporter ions. Molecular & Cellular Proteomics. doi:10.1074/mcp.M112.025569.
Lattante, S., Rouleau, G., & Kabashi, E. (2013). TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: Summary and update. Human Mutation, 34, 812–826.
Lin, D. Y., Huang, Y. S., Jeng, J. C., & Shih, H. M. (2006). Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Molecular Cell, 24, 341–354.
Mahajan, R., Gerace, L., & Melchior, F. (1998). Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. Journal of Cell Biology, 140, 259–270.
Martin, S., Nishimune, A., Mellor, J. R., & Henley, J. M. (2007). SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature, 447, 321–325.
Matafora, V., D’Amato, A., Mori, S., Blasi, F., & Bachi, A. (2009). Proteomics analysis of nucleolar SUMO-1 target proteins upon proteasome inhibition. Molecular and Cellular Proteomics, 8, 2243–2255.
Matic, I., Schimmel, J., Hendriks, I., Van Santen, M., Van de Rijke, F., Van Dam, H., et al. (2010). Site-specific identification of SUMO-2 targets in cells reveals an inverted SUMOylation motif and a hydrophobic cluster SUMOylation motif. Molecular Cell, 39, 641–652.
Melchior, F., & Hengst, L. (2002). SUMO-1 and p53. Cell Cycle, 1, 245–249.
Meulmeester, E., & Melchior, F. (2008). Cell biology: SUMO. Nature, 452, 709–711.
Minty, A., Dumont, X., Kaghad, M., & Caput, D. (2000). Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. Journal of Biological Chemistry, 275, 36316–36323.
Mooney, S. M., Grande, J. P., Salisbury, J. L., & Janknecht, R. (2010). Sumoylation of p68 and p72 RNA helicases affects protein stability and transactivation potential. Biochemistry, 49, 1–10.
Morlando, M., Modigliani, S. D., Torrelli, G., Rosa, A., Di Carlo, V., Caffarelli, E., et al. (2012). FUS stimulates microRNA biogenesis by facilitating co-transcriptional Drosha recruitment. EMBO Journal, 31, 4502–4510.
Morris, J. R., Boutell, C., Keppler, M., Densham, R., & Solomon, E. (2009). The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature, 462, 886–890.
Moumen, A., Masterson, P., O’Connor, M. J., & Jackson, S. P. (2005). hnRNP K: An HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell, 123, 1065–1078.
Mukhopadhyay, D., & Dasso, M. (2007). Modification in reverse: The SUMO proteases. Trends in Biochemical Sciences, 32, 286–295.
Müller, S., Matunis, M. J., & Dejean, A. (1998). Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO Journal, 17, 61–70.
Navascues, J., Bengoechea, R., Tapia, O., Casafont, I., Berciano, M. T., & Lafarga, M. (2008). SUMO-1 transiently localizes to Cajal bodies in mammalian neurons. Journal of Structural Biology, 163, 137–146.
Oh, S. M., Liu, Z., Okada, M., Jang, S. W., Liu, X., Chan, C. B., et al. (2010). Ebp1 sumoylation, regulated by TLS/FUS E3 ligase, is required for its anti-proliferative activity. Oncogene, 29, 1017–1030.
Ong, S. E. (2002). Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Molecular and Cellular Proteomics, 1, 376–386.
Osula, O., Swatkoski, S., & Cotter, R. J. (2012). Identification of protein SUMOylation sites by mass spectrometry using combined microwave-assisted aspartic acid cleavage and tryptic digestion. Journal of Mass Spectrometry, 47, 644–654.
Pedrioli, P. G. A., Raught, B., Zhang, X., Rogers, R., Aitchison, J., Matunis, M., et al. (2006). Automated identification of SUMOylation sites using mass spectrometry and SUMmOn pattern recognition software. Nature Methods, 3, 533–539.
Pedrotti, S., Bielli, P., Paronetto, M. P., Ciccosanti, F., Fimia, G. M., Stamm, S., et al. (2010). The splicing regulator Sam68 binds to a novel exonic splicing silencer and functions in SMN2 alternative splicing in spinal muscular atrophy. The EMBO Journal, 29, 1235–1247.
Pelisch, F., Gerez, J., Druker, J., Schor, I. E., Muñoz, M. J., Risso, G., et al. (2010). The serine/arginine-rich protein SF2/ASF regulates protein sumoylation. Proceedings of the National Academy of Sciences of the United States of America, 107, 16119–16124.
Rajan, S., Plant, L. D., Rabin, M. L., Butler, M. H., & Goldstein, S. A. (2005). Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell, 121, 37–47.
Ren, J., Gao, X., Jin, C., Zhu, M., Wang, X., Shaw, A., et al. (2009). Systematic study of protein sumoylation: Development of a site-specific predictor of SUMOsp 2.0. Proteomics, 9, 3409–3412.
Rigaut, G., Shevchenko, A., Rutz, B., Wilm, M., Mann, M., & Séraphin, B. (1999). A generic protein purification method for protein complex characterization and proteome exploration. Nature Biotechnology, 17, 7–9.
Rosas-Acosta, G., Russell, W. K., Deyrieux, A., Russell, D. H., & Wilson, V. G. (2005). A universal strategy for proteomic studies of SUMO and other ubiquitin-like modifiers. Molecular & Cellular Proteomics, 56–72.
Russell, R., Jarmoskaite, I., & Lambowitz, A. M. (2013). Toward a molecular understanding of RNA remodeling by DEAD-box proteins. RNA Biology, 10, 44–55.
Saitoh, H., & Hinchey, J. (2000). Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. Journal of Biological Chemistry, 275, 6252–6258.
Sampson, D., Wang, M., & Matunis, M. J. (2001). Ubc9 binding and is essential for SUMO-1 modification. Journal of Biological Chemistry, 276, 21664–21669.
Sansam, C. L., Wells, K. S., & Emeson, R. B. (2003). Modulation of RNA editing by functional nucleolar sequestration of ADAR2. Proceedings of the National Academy of Sciences of the United States of America, 100, 14018–14023.
Schimmel, J., Larsen, K. M., Matic, I., Van Hagen, M., Cox, J., Mann, M., et al. (2008). The ubiquitin-proteasome system is a key component of the SUMO-2/3 cycle. Molecular and Cellular Proteomics, 7, 2107–2122.
Seeler, J. S., & Dejean, A. (2003). Nuclear and unclear functions of SUMO. Nature Reviews Molecular Cell Biology, 4, 690–699.
Sette, C. (2010). Post-translational regulation of star proteins and effects on their biological functions. Advances in Experimental Medicine and Biology, 693, 54–66.
Seyfried, N. T., Gozal, Y. M., Dammer, E. B., Xia, Q., Duong, D. M., Cheng, D., et al. (2010). Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains. Molecular and Cellular Proteomics, 9, 705–718.
Shinbo, Y., Niki, T., Taira, T., Ooe, H., Takahashi-Niki, K., Maita, C., et al. (2006). Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death and Differentiation, 13, 96–108.
Song, J., Durrin, L. K., Wilkinson, T., Krontiris, T. G., & Chen, Y. (2004). Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proceedings of the National Academy of Sciences of the United States of America, 101, 14373–14378.
Steen, H., & Mann, M. (2004). The ABC’s (and XYZ’s) of peptide sequencing. Nature Reviews Molecular Cell Biology, 5, 699–711.
Stehmeier, P., & Muller, S. (2009a). Phospho-regulated SUMO interaction modules connect the SUMO system to CK2 signaling. Molecular Cell, 33, 400–409.
Stehmeier, P., & Muller, S. (2009b). Regulation of p53 family members by the ubiquitin-like SUMO system. DNA Repair Amst, 8, 491–498.
Sternsdorf, T., Jensen, K., Reich, B., & Will, H. (1999). The nuclear dot protein sp100, characterization of domains necessary for dimerization, subcellular localization, and modification by small ubiquitin-like modifiers. Journal of Biological Chemistry, 274, 12555–12566.
Tago, K., Chiocca, S., & Sherr, C. J. (2005). Sumoylation induced by the Arf tumor suppressor: A p53-independent function. Proceedings of the National Academy of Sciences of the United States of America, 102, 7689–7694.
Tang, Z., El Far, O., Betz, H., & Scheschonka, A. (2005). Pias1 interaction and sumoylation of metabotropic glutamate receptor 8. Journal of Biological Chemistry, 280, 38153–38159.
Tatham, M. H., Rodriguez, M. S., Xirodimas, D. P., & Hay, R. T. (2009). Detection of protein SUMOylation in vivo. Nature Protocols, 4, 1363–1371.
Taylor, S. J., Resnick, R. J., & Shalloway, D. (2004). Sam68 exerts separable effects on cell cycle progression and apoptosis. BMC Cell Biology, 5, 5.
Tirard, M., Hsiao, H.-H., Nikolov, M., Urlaub, H., Melchior, F., & Brose, N. (2012). In vivo localization and identification of SUMOylated proteins in the brain of His6-HA-SUMO1 knock-in mice. Proceedings of the National Academy of Sciences of the United States of America, 109, 21122–21127.
Van Niekerk, E., Willis, D. E., Chang, J. H., Reumann, K., Heise, T., & Twiss, J. L. (2007). Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proceedings of the National Academy of Sciences of the United States of America, 104, 12913–12918.
Vassileva, M. T., Matunis, M. J., Vassileva, M. T., & Matunis, M. J. (2004). SUMO modification of heterogeneous nuclear ribonucleoproteins SUMO modification of heterogeneous nuclear ribonucleoproteins. Molecular and Cellular Biology, 24, 3623–3632.
Vertegaal, A. C. O., Andersen, J. S., Ogg, S. C., Hay, R. T., Mann, M., & Lamond, A. I. (2006). Distinct and overlapping sets of SUMO-1 and SUMO-2 target proteins revealed by quantitative proteomics. Molecular and Cellular Proteomics, 5, 2298–2310.
Vertegaal, A. C., Ogg, S. C., Jaffray, E., Rodriguez, M. S., & Lamond, A. I. (2004). A proteomic study of SUMO-2 target proteins. Journal of Biological Chemistry, 279, 33791–33798.
Wagner, S. A., Beli, P., Weinert, B. T., Nielsen, M. L., Mann, M., & Choudhary C. (2011). A proteome-wide quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Molecular & Cellular Proteomics, 10, M111.013284.
Wahle, S., Rohweder, H., Ribbe, J., & Steinert, K. (1999). Purification of 6xHis-tagged proteins from mammalian expression systems using Ni-NTA magnetic agarose beads. QIAGEN News, 1999(4), 3.
Westman, B. J., & Lamond, A. I. (2011). A role for SUMOylation in snoRNP biogenesis revealed by quantitative proteomics. Nucleus, 2, 30–37.
Westman, B. J., Verheggen, C., Hutten, S., Lam, Y. W., Bertrand, E., & Lamond, A. I. (2010). A proteomic screen for nucleolar SUMO targets shows SUMOylation modulates the function of Nop5/Nop58. Molecular Cell, 39, 618–631.
Wilkinson, K., & Henley, J. M. (2010). Mechanisms, regulation and consequences of protein SUMOylation. Biochemistry Journal, 428, 133–145.
Wolin, S. L., & Cedervall, T. (2002). The La protein. Annual Review of Biochemistry, 71, 375–403.
Xu, J., He, Y., Qiang, B., Yuan, J., Peng, X., & Pan, X.-M. (2008). A novel method for high accuracy sumoylation site prediction from protein sequences. BMC Bioinformatics, 8, 8.
Xu, G., Paige, J. S., & Jaffrey, S. R. (2010). Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nature Biotechnology, 28, 868–873.
Yang, S. H., Galanis, A., Witty, J., & Sharrocks, A. D. (2006). An extended consensus motif enhances the specificity of substrate modification by SUMO. EMBO Journal, 25, 5083–5093.
Yang, C., Maiguel, D., & Carrier, F. (2002). Identification of nucleolin and nucleophosmin as genotoxic stress-responsive RNA-binding proteins. Nucleic Acids Research, 30, 2251–2260.
Zeng, L., Yap, K. L., Ivanov, A. V., Wang, X., Mujtaba, S., Plotnikova, O., et al. (2008). Structural insights into human KAP1 PHD finger–bromodomain and its role in gene silencing. Nature Structural & Molecular Biology, 15, 626–633.
Zhao, Y., Kwon, S. W., Anselmo, A., Kaur, K., & White, M. (2004). Broad spectrum identification of cellular small ubiquitin-related modifier (SUMO) substrate proteins. Journal of Biological Chemistry, 279, 20999–21002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filosa, G., Barabino, S.M.L. & Bachi, A. Proteomics Strategies to Identify SUMO Targets and Acceptor Sites: A Survey of RNA-Binding Proteins SUMOylation. Neuromol Med 15, 661–676 (2013). https://doi.org/10.1007/s12017-013-8256-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-013-8256-8