Abstract
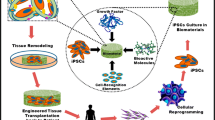
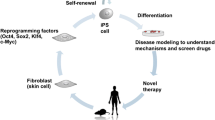
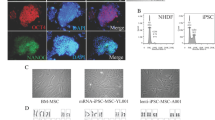
Induced pluripotent stem (iPS) cells, possess high proliferation and differentiation ability, are now considered an attractive option for osteogenic differentiation and bone regeneration. In fact, recent discoveries have demonstrated that iPS cells can be differentiated into osteoblasts, suggesting that iPS cells have the potential to advance future bone regenerative therapies. Herein, we provide an overview of the recent findings on osteogenic characteristics and differentiation potential of iPS cells. In addition, we discuss current methods for inducing their specification towards osteogenic phenotype as well as in vivo evidence supporting the therapeutic benefit of iPS-derived osteoblasts. Finally, we describe recent findings regarding the use of iPS-derived cells for osteogenic differentiation and bone regeneration, which have indicated that these pluripotent cells represent an ideal tool for regenerative cell therapies and might contribute to the development of future bone tissue engineering.
Similar content being viewed by others
References
Sabareeswaran, A., Basu, B., Shenoy, S. J., Jaffer, Z., Saha, N., & Stamboulis, A. (2013). Early osseointegration of a strontium containing glass ceramic inarabbit model. Biomaterials, 34(37), 9278–9286.
O’Keefe, R. J., & Mao, J. (2011). Bone tissue engineering and regeneration: from discovery to the clinic-an overview introduction. Tissue Engineering, Part B-Reviews, 17(6), 389–392.
Nawawi, N. A., Alqap, A. S. F., & Sopyan, I. (2011). Recent progress on hydroxyapatite-based dense biomaterials for load bearing bone substitutes. Recent Pat Mater Science, 4(1), 63–80.
Tan, L., Yu, X., Wan, P., & Yang, K. (2013). Biodegradable materials for bone repairs: a review. Journal of Materials Science and Technology, 29(6), 503–513.
Murphy, S. V., & Atala, A. (2013). Organ engineering combining stem cells, biomaterials, and bioreactors to produce bioengineered organs for transplantation. Bioessays, 35(3), 163–172.
Martino, S., D’Angelo, F., Armentano, I., Maria Kenny, J., & Orlacchio, A. (2012). Stem cell-biomaterial interactions for regenerative medicine. Biotechnology Advances, 30(1), 338–351.
Prabhakaran, M. P., Venugopal, J., Ghasemi-Mobarakeh, L., Kai. D., Jin, G., Ramakrishna. S., (2012). Stem Cells and Nanostructures for Advanced Tissue Regeneration. In: Biomedical Applications of Polymeric Nanofibers. Edited by Jayakumar R, Nair SV, vol. 246: 21–62.
Liu, H., Peng, H., Wu, Y., Zhang, C., Cai, Y., Xu, G., Li, Q., Chen, X., Ji, J., Zhang, Y., et al. (2013). The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials, 34(18), 4404–4417.
Peng, H., Yin, Z., Liu, H., Chen, X., Feng, B., Yuan, H., Su, B., Ouyang, H., Zhang, Y. (2012). Electrospun biomimetic scaffold of hydroxyapatite/chitosan supports enhanced osteogenic differentiation of mMSCs. Nanotechnology, 23(48).
Yoshida, Y., & Yamanaka, S. (2011). iPS cells: a source of cardiac regeneration. Journal of Molecular and Cellular Cardiology, 50(2), 327–332.
Stenderup, K., Justesen, J., Clausen, C., & Kassem, M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone, 33(6), 919–926.
Wen, Y., Wang, F., Zhang, W., Li, Y., Yu, M., Nan, X., Chen, L., Yue, W., Xu, X., & Pei, X. (2012). Application of induced pluripotent stem cells in generation of a tissue-engineered tooth-like structure. Tissue Engineering Part A, 18(15–16), 1677–1685.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318(5858), 1917–1920.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Takahashi, K., & Yamanaka, S. (2013). Induced pluripotent stem cells in medicine and biology. Development, 140(12), 2457–2461.
Yamanaka, S. (2012). Induced pluripotent stem cells: past, present, and future. Cell Stem Cell, 10(6), 678–684.
Teng, S., Liu, C., Krettek, C., & Jagodzinski, M. (2014). The application of induced pluripotent stem cells for bone regeneration: current progress and prospects. Tissue Eng, Part B-Rev, 20(4), 328–339.
Shen, H.-F., Yao, Z.-F., Xiao, G.-F., Jia, J.-S., Xiao, D., & Yao, K.-T. (2009). Induced pluripotent stem cells (iPS Cells): current status and future prospect. Progress in Biochemistry and Biophysics, 36(8), 950–960.
Duan, X., Tu, Q., Zhang, J., Ye, J., Sommer, C., Mostoslavsky, G., Kaplan, D., Yang, P., & Chen, J. (2011). Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. Journal of Cellular Physiology, 226(1), 150–157.
Nelson, T. J., Martinez-Fernandez, A., & Terzic, A. (2010). Induced pluripotent stem cells: developmental biology to regenerative medicine. Nature Reviews Cardiology, 7(12), 700–710.
Iglesias-Garcia, O., Pelacho, B., & Prosper, F. (2013). Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling. Journal of Molecular and Cellular Cardiology, 62, 43–50.
Ardeshirylajimi, A., & Soleimani, M. (2015). Enhanced growth and osteogenic differentiation of induced pluripotent stem cells by extremely Low-frequency electromagnetic field. Cellular and Molecular Biology, 61(1), 36–41.
Wang, M., Deng, Y., Zhou, P., Luo, Z., Li, Q., Xie, B., Zhang, X., Chen, T., Pei, D., Tang, Z., et al. (2015). In vitro culture and directed osteogenic differentiation of human pluripotent stem cells on peptides-decorated two-dimensional microenvironment. ACS Applied Materials & Interfaces, 7(8), 4560–4572.
Kawaguchi, J. (2006). Generation of osteoblasts and chondrocytes from embryonic stem cells. Methods in Molecular Biology (Clifton, NJ), 330, 135–148.
Grassel, S., Stockl, S., & Jenei-Lanzl, Z. (2012). Isolation, culture, and osteogenic/chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Methods in Molecular Biology (Clifton, NJ), 879, 203–267.
Alfred, R., Taiani, J. T., Krawetz, R. J., Yamashita, A., Rancourt, D. E., & Kallos, M. S. (2011). Large-scale production of murine embryonic stem cell-derived osteoblasts and chondrocytes on microcarriers in serum-free media. Biomaterials, 32(26), 6006–6016.
Lavrentieva, A., Hatlapatka, T., Neumann, A., Weyand, B., & Kasper, C. (2013). Potential for osteogenic and chondrogenic differentiation of MSC. In B. Weyand, M. Dominici, R. Hass, R. Jacobs, & C. Kasper (Eds.), Mesenchymal stem cells: Basics and clinical application I (Vol. 129, pp. 73–88).
Kumaran, S. T., Arun, K. V., Sudarsan, S., Talwar, A., & Srinivasan, N. (2010). Osteoblast response to commercially available demineralized bone matrices–an in-vitro study. Indian Journal of Dental Research : Official Publication of Indian Society for Dental Research, 21(1), 3–9.
Hayashi, T., Misawa, H., Nakahara, H., Noguchi, H., Yoshida, A., Kobayashi, N., Tanaka, M., & Ozaki, T. (2012). Transplantation of osteogenically differentiated mouse iPS cells for bone repair. Cell Transplantation, 21(2–3), 591–600.
Quarto, N., Li, S., Renda, A., & Longaker, M. T. (2012). Exogenous activation of BMP-2 signaling overcomes TGF beta-mediated inhibition of osteogenesis in marfan embryonic stem cells and marfan patient-specific induced pluripotent stem cells. Stem Cells, 30(12), 2709–2719.
zur Nieden, N. I., Kempka, G., & Ahr, H. J. (2003). In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation, 71(1), 18–27.
Rui, Y. F., Lui, P. P. Y., Ni, M., Chan, L. S., Lee, Y. W., & Chan, K. M. (2011). Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. Journal of Orthopaedic Research, 29(3), 390–396.
Zachos, T. A., Shields, K. M., & Bertone, A. L. (2006). Gene-mediated osteogenic differentiation of stem cells by bone morphogenetic proteins-2 or-6. Journal of Orthopaedic Research, 24(6), 1279–1291.
Song, I., Kim, B.-S., Kim, C.-S., & Im, G.-I. (2011). Effects of BMP-2 and vitamin D-3 on the osteogenic differentiation of adipose stem cells. Biochemical and Biophysical Research Communications, 408(1), 126–131.
Luu, H. H., Song, W. X., Luo, X. J., Manning, D., Luo, J. Y., Deng, Z. L., Sharffl, K. A., Montag, A. G., Haydon, R. C., & He, T. C. (2007). Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. Journal of Orthopaedic Research, 25(5), 665–677.
Kao, C.-L., Tai, L.-K., Chiou, S.-H., Chen, Y.-J., Lee, K.-H., Chou, S.-J., Chang, Y.-L., Chang, C.-M., Chen, S.-J., Ku, H.-H., et al. (2010). Resveratrol promotes osteogenic differentiation and protects against Dexamethasone damage in murine induced pluripotent stem cells. Stem Cells and Development, 19(2), 247–258.
Okano, H., Nakamura, M., Yoshida, K., Okada, Y., Tsuji, O., Nori, S., Ikeda, E., Yamanaka, S., & Miura, K. (2013). Steps toward safe cell therapy using induced pluripotent stem cells. Circulation Research, 112(3), 523–533.
Li, F., Niyibizi, C., (2012). Cells derived from murine induced pluripotent stem cells (iPSC) by treatment with members of TGF-beta family give rise to osteoblasts differentiation and form bone in vivo. Bmc Cell Biology, 13.
Bilousova, G., Jun, D. H., King, K. B., De Langhe, S., Chick, W. S., Torchia, E. C., Chow, K. S., Klemm, D. J., Roop, D. R., & Majka, S. M. (2011). Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells, 29(2), 206–216.
Tashiro, K., Inamura, M., Kawabata, K., Sakurai, F., Yamanishi, K., Hayakawa, T., & Mizuguchi, H. (2009). Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells, 27(8), 1802–1811.
Stefani, G., & Slack, F. J. (2008). Small non-coding RNAs in animal development. Nature Reviews Molecular Cell Biology, 9(3), 219–230.
Li, Z., Hassan, M. Q., Volinia, S., van Wijnen, A. J., Stein, J. L., Croce, C. M., Lian, J. B., & Stein, G. S. (2008). A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proceedings of the National Academy of Sciences of the United States of America, 105(37), 13906–13911.
Li, Z., Hassan, M. Q., Jafferji, M., Aqeilan, R. I., Garzon, R., Croce, C. M., van Wijnen, A. J., Stein, J. L., Stein, G. S., & Lian, J. B. (2009). Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. Journal of Biological Chemistry, 284(23), 15676–15684.
Okamoto, H., Matsumi, Y., Hoshikawa, Y., Takubo, K., Ryoke, K., Shiota, G., (2012). Involvement of MicroRNAs in Regulation of Osteoblastic Differentiation in Mouse Induced Pluripotent Stem Cells. Plos One, 7(8).
Jin, G.-Z., Kim, T.-H., Kim, J.-H., Won, J.-E., Yoo, S.-Y., Choi, S.-J., Hyun, J. K., & Kim, H.-W. (2013). Bone tissue engineering of induced pluripotent stem cells cultured with macrochanneled polymer scaffold. Journal of Biomedical Materials Research. Part A, 101A(5), 1283–1291.
Chen, X.-D., Dusevich, V., Feng, J. Q., Manolagas, S. C., & Jilka, R. L. (2007). Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. Journal of Bone and Mineral Research, 22(12), 1943–1956.
Holzwarth, J. M., & Ma, P. X. (2011). Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials, 32(36), 9622–9629.
D’Angelo, F., Armentano, I., Cacciotti, I., Tiribuzi, R., Quattrocelli, M., Del Gaudio, C., Fortunati, E., Saino, E., Caraffa, A., Cerulli, G. G., et al. (2012). Tuning multi/pluri-potent stem cell fate by electrospun poly(L-lactic acid)-calcium-deficient hydroxyapatite nanocomposite mats. Biomacromolecules, 13(5), 1350–1360.
Kobayashi, T., Yamaguchi, T., Hamanaka, S., Kato-Itoh, M., Yamazaki, Y., Ibata, M., Sato, H., Lee, Y.-S., Usui, J.-i., Knisely, A. S., et al. (2010). Generation of Rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell, 142(5), 787–799.
Nelson, T. J., Martinez-Fernandez, A., Yamada, S., Perez-Terzic, C., Ikeda, Y., & Terzic, A. (2009). Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation, 120(5), 408–416.
Park, S., & Im, G.-I. (2014). Embryonic stem cells and induced pluripotent stem cells for skeletal regeneration. Tissue English, Part B-Rev, 20(5), 381–391.
Polo, J. M., Liu, S., Figueroa, M. E., Kulalert, W., Eminli, S., Tan, K. Y., Apostolou, E., Stadtfeld, M., Li, Y. S., Shioda, T., et al. (2010). Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature Biotechnology, 28(8), 848–U130.
Nasu, A., Ikeya, M., Yamamoto, T., Watanabe, A., Jin, Y. H., Matsumoto, Y., Hayakawa, K., Amano, N., Sato, S., Osafune, K. et al, (2013). Genetically Matched Human iPS Cells Reveal that Propensity for Cartilage and Bone Differentiation Differs with Clones, not Cell Type of Origin. Plos One, 8(1).
Robinton, D. A., & Daley, G. Q. (2012). The promise of induced pluripotent stem cells in research and therapy. Nature, 481(7381), 295–305.
Feng, B., Ng, J. H., Heng, J. C. D., & Ng, H. H. (2009). Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell, 4(4), 301–312.
Acknowledgments
This contribution is funded by the Natural Science Foundation Project of Shanghai, China (15ZR1400500) and the Fundamental Research Funds for the Central Universities by the Ministry of Education of China (2232013D3-13 and 15D110538).
Conflict of Interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lou, X. Induced Pluripotent Stem Cells as a new Strategy for Osteogenesis and Bone Regeneration. Stem Cell Rev and Rep 11, 645–651 (2015). https://doi.org/10.1007/s12015-015-9594-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-015-9594-8