Abstract
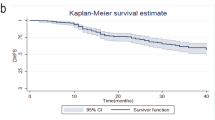
The study aims to identify clinical and pathological factors predictive of disease-free survival (DFS) and overall survival (OS) in locally advanced breast cancer (LABC) patients who do not have a pathologic complete response (no-pCR) of primary tumor after neoadjuvant chemotherapy (NC) with vinorelbine/epirubicin (VE) intravenous combination regimen. Retrospectively reviewed data of LABC patients in our Hospital. 97 patients who had no-pCR after NC were identified and enrolled in the study. All patients were treated with three cycles of VE intravenous administration before operation. Local–regional radiotherapy was offered to patients after the completion of chemotherapy followed by hormone therapy according to hormone receptor status. Neoadjuvant chemotherapy consisting of intravenous vinorelbine 25 mg/m on day 1 and 8 plus epirubicin 60 mg/m on day 1 was administered every 3 weeks. The relationship of survival with clinical and pathological factors was evaluated. Univariate analysis (log-rank tests) and multivariate analysis (Cox regression analysis) were performed to identify independent predictors for DFS and OS. Study was analyzed with a median follow-up of 65 months. The 5-year rates for DFS and OS were 58.0 and 68.5 %, respectively. Multivariate analysis revealed that three factors such as the estrogen receptor expression before NC (pre-ER), Ki-67 expression after NC (post-Ki-67), and pathological response of primary tumor (pRT) were independent prognostic factors of LABC patients (pre-ER and pRT for DFS, all three for OS). The DFS at 5 years was 73.8 % for patients without both factors, 51.5 % for patients with any one of both factors, and 10.3 % for patients with both factors. The OS at 5 years was 90.5 % for patients without these three factors, 64.3 % for patients with any one of these three factors, and 30.8 % for patients with any two of these three factors. Patients with all three factors died within 3 years. In LABC patients with no-pCR, three factors independently predicted of survival and, without those three high-risk factors, patients had the promising outcome.


Similar content being viewed by others
References
Aktas, B., Muller, V., Tewes, M., Zeitz, J., Kasimir-Bauer, S., Loehberg, C. R., et al. (2011). Comparison of estrogen and progesterone receptor status of circulating tumor cells and the primary tumor in metastatic breast cancer patients. Gynecologic Oncology, 122, 356–360.
Mathieu, M. C., Mazouni, C., Kesty, N. C., Zhang, Y., Scott, V., Passeron, J., et al. (2012). Breast Cancer Index predicts pathological complete response and eligibility for breast conserving surgery in breast cancer patients treated with neoadjuvant chemotherapy. Annals of Oncology, 23, 2046–2052.
Miglietta, L., Vanella, P., Canobbio, L., Naso, C., Cerisola, N., Meszaros, P., et al. (2010). Prognostic value of estrogen receptor and Ki-67 index after neoadjuvant chemotherapy in locally advanced breast cancer expressing high levels of proliferation at diagnosis. Oncology, 79, 255–261.
Oliveira, R. F., Dos, S. R., de Oliveira, A. L., de Lima, R. R., de Melo, M. B., & Scheffer, D. K. (2012). Prognostic assessment of polymorphisms of the MDR-1 and GSTP1 genes in patients with stage II and III breast cancer submitted to neoadjuvant chemotherapy. Breast Journal, 18, 185–187.
Takada, M., Higuchi, T., Tozuka, K., Takei, H., Haruta, M., Watanabe, J., et al. (2013). Alterations of the genes involved in the PI3K and estrogen-receptor pathways influence outcome in human epidermal growth factor receptor 2-positive and hormone receptor-positive breast cancer patients treated with trastuzumab-containing neoadjuvant chemotherapy. BMC Cancer, 13, 241.
Wang, Y., Sparano, J. A., Fineberg, S., Stead, L., Sunkara, J., Horwitz, S. B., et al. (2013). High expression of class III beta-tubulin predicts good response to neoadjuvant taxane and doxorubicin/cyclophosphamide-based chemotherapy in estrogen receptor-negative breast cancer. Clinical Breast Cancer, 13, 103–108.
Nakagawa, T., Sato, K., Moriwaki, M., Wada, R., Arakawa, A., Saito, M., et al. (2012). Successful endocrine therapy for locally advanced mucinous carcinoma of the breast. Breast Journal, 18, 632–633.
Wang, J., Buchholz, T. A., Middleton, L. P., Allred, D. C., Tucker, S. L., Kuerer, H. M., et al. (2002). Assessment of histologic features and expression of biomarkers in predicting pathologic response to anthracycline-based neoadjuvant chemotherapy in patients with breast carcinoma. Cancer, 94, 3107–3114.
Amini, A., Kakkis, J., Reitherman, R., Ibarra, J., & Sanati, H. (2013). Near-complete pathological response with preoperative chemotherapy in a patient with metaplastic breast carcinoma. Anti-Cancer Drugs, 24, 765–768.
van der Hage, J. A., van de Velde, C. J., Julien, J. P., Tubiana-Hulin, M., Vandervelden, C., & Duchateau, L. (2001). Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. Journal of Clinical Oncology, 19, 4224–4237.
Bear, H. D., Anderson, S., Smith, R. E., Geyer, C. J., Mamounas, E. P., Fisher, B., et al. (2006). Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. Journal of Clinical Oncology, 24, 2019–2027.
Makhoul, I., Klimberg, V. S., Korourian, S., Henry-Tillman, R. S., Siegel, E. R., Westbrook, K. C., et al. (2013). Combined neoadjuvant chemotherapy with bevacizumab improves pathologic complete response in patients with hormone receptor negative operable or locally advanced breast cancer. American Journal of Clinical Oncology. doi: 10.1097/COC.0b013e31828940c3.
Conti, F., & Vici, P. (1998). Vinorelbin in the treatment of breast cancer: current status and prospectives for the future. Clinica Terapeutica, 149, 61–74.
Spielmann, M., Dorval, T., Turpin, F., Antoine, E., Jouve, M., Maylevin, F., et al. (1994). Phase II trial of vinorelbine/doxorubicin as first-line therapy of advanced breast cancer. Journal of Clinical Oncology, 12, 1764–1770.
Norris, B., Pritchard, K. I., James, K., Myles, J., Bennett, K., Marlin, S., et al. (2000). Phase III comparative study of vinorelbine combined with doxorubicin versus doxorubicin alone in disseminated metastatic/recurrent breast cancer: National Cancer Institute of Canada Clinical Trials Group Study MA8. Journal of Clinical Oncology, 18, 2385–2394.
Chen, C. M., Shen, K. W., Liu, G. Y., Wu, J., Lu, J. S., Zhuang, C. J., et al. (2006). A study of the combination of vinorelbine and epirubicin as neoadjuvant chemotherapy regimen in the treatment of locally advanced breast cancer. Zhonghua Wai Ke Za Zhi, 44, 745–747.
Wenners, A. S., Mehta, K., Loibl, S., Park, H., Mueller, B., Arnold, N., et al. (2012). Neutrophil gelatinase-associated lipocalin (NGAL) predicts response to neoadjuvant chemotherapy and clinical outcome in primary human breast cancer. PLoS ONE, 7, e45826.
Hammond, M. E., Hayes, D. F., Dowsett, M., Allred, D. C., Hagerty, K. L., Badve, S., et al. (2010). American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of Clinical Oncology, 28, 2784–2795.
Wolff, A. C., Hammond, M. E., Schwartz, J. N., Hagerty, K. L., Allred, D. C., Cote, R. J., et al. (2007). American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Journal of Clinical Oncology, 25, 118–145.
Singletary, S. E., Allred, C., Ashley, P., Bassett, L. W., Berry, D., Bland, K. I., et al. (2002). Revision of the American Joint Committee on Cancer staging system for breast cancer. Journal of Clinical Oncology, 20, 3628–3636.
Ayers, M., Symmans, W. F., Stec, J., Damokosh, A. I., Clark, E., Hess, K., et al. (2004). Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. Journal of Clinical Oncology, 22, 2284–2293.
Chang, J. C., Wooten, E. C., Tsimelzon, A., Hilsenbeck, S. G., Gutierrez, M. C., Elledge, R., et al. (2003). Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet, 362, 362–369.
Taucher, S., Rudas, M., Gnant, M., Thomanek, K., Dubsky, P., Roka, S., et al. (2003). Sequential steroid hormone receptor measurements in primary breast cancer with and without intervening primary chemotherapy. Endocrine Related Cancer, 10, 91–98.
Makris, A., Powles, T. J., Allred, D. C., Ashley, S. E., Trott, P. A., Ormerod, M. G., et al. (1999). Quantitative changes in cytological molecular markers during primary medical treatment of breast cancer: a pilot study. Breast Cancer Research and Treatment, 53, 51–59.
Burcombe, R., Wilson, G. D., Dowsett, M., Khan, I., Richman, P. I., Daley, F., et al. (2006). Evaluation of Ki-67 proliferation and apoptotic index before, during and after neoadjuvant chemotherapy for primary breast cancer. Breast Cancer Research, 8, R31.
Dowsett, M., Ebbs, S. R., Dixon, J. M., Skene, A., Griffith, C., Boeddinghaus, I., et al. (2005). Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer–a study from the IMPACT trialists. Journal of Clinical Oncology, 23, 2477–2492.
Dowsett, M., Smith, I. E., Ebbs, S. R., Dixon, J. M., Skene, A., Griffith, C., et al. (2005). Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clinical Cancer Research, 11, 951s–958s.
Ferguson NL, Bell J, Heidel R, Lee S, Vanmeter S, Duncan L, Munsey B, Panella T, Orucevic A. (2013). Prognostic value of breast cancer subtypes, Ki-67 proliferation index, age, and pathologic tumor characteristics on breast cancer survival in Caucasian women. Breast Journal, 19, 22–30.
Lee, J., Im, Y. H., Lee, S. H., Cho, E. Y., Choi, Y. L., Ko, Y. H., et al. (2008). Evaluation of ER and Ki-67 proliferation index as prognostic factors for survival following neoadjuvant chemotherapy with doxorubicin/docetaxel for locally advanced breast cancer. Cancer Chemotherapy and Pharmacology, 61, 569–577.
Wiesner, F. G., Magener, A., Fasching, P. A., Wesse, J., Bani, M. R., Rauh, C., et al. (2009). Ki-67 as a prognostic molecular marker in routine clinical use in breast cancer patients. Breast, 18, 135–141.
Billgren, A. M., Rutqvist, L. E., Tani, E., Wilking, N., Fornander, T., & Skoog, L. (1999). Proliferating fraction during neoadjuvant chemotherapy of primary breast cancer in relation to objective local response and relapse-free survival. Acta Oncologica, 38, 597–601.
Acknowledgments
We thank all the surgeons, radiologists, oncologists, pathologists, and nurses who contributed; Ya-fen Li, Wei-guo Chen for assistance with this study. This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81172520; 81202087; 81202088, 81472462), Natural Science Foundation of Shanghai Municipal Science and Technology Commission (Grant Number: 12ZR1446400), Technology Innovation Act Plan of Shanghai Municipal Science and Technology Commission (Grant Number: 14411950200, 14411950201), and Joint Research Project of the Emerging Cutting-edge Technology of Shanghai Shen-kang Hospital Development Center (Grant Number: SHDC12014103).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, O., Jiang, M., Chen, Xs. et al. Prognostic Factors of Survival in Pathologic Incomplete Response Patients with Locally Advanced Breast Cancer After Neoadjuvant Chemotherapy. Cell Biochem Biophys 71, 1181–1190 (2015). https://doi.org/10.1007/s12013-014-0327-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0327-4