Abstract
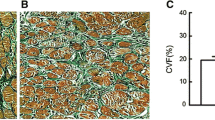
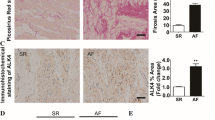
Patients with rheumatic heart disease (RHD) often experience persistent atrial fibrillation (AF) associated with adverse atrial structural remodeling (ASR) manifested by atrial fibrosis and left atrial enlargement. The aim of this study was to explore the potential molecular signaling mechanisms for atrial fibrosis and ASR. Twenty RHD patients with persistent AF and 10 RHD patients with sinus rhythm (Group A) were recruited in our study, which all underwent transthoracic echocardiography. Right atrial appendage (RAA) tissue samples were obtained from these patients during mitral/aortic valve replacement operation. The AF patients were further divided into two groups according to left atrial diameter (LAD): Group B with LAD ranging 50–65 mm and Group C with LAD >65 mm. Histological examinations were performed with hematoxylin–eosin staining and Masson’s trichrome staining. Atrial angiotensin II (AngII) content was measured by ELISA. Rac1 and STAT3 protein levels were determined by Western blot analysis. Hematoxylin–eosin staining demonstrated highly organized arrangement of atrial muscles in control Group A and significant derangement in both Group B and C AF patients with reduced cell density and increased cell size. Moreover, Masson’s trichrome staining showed that atrial myocytes were surrounded by large trunks of collagen fibers in both Group B and C, but not in Group A. There was a positive correlation between atrial tissue fibrosis and LAD. AngII content was markedly higher in Group C than in Group B than in Group A, which was positively correlated with LAD. Similarly, Rac1 and STAT3 protein levels were found considerably higher in Group C and B than in Group A with excellent correlation to LAD. Our study unraveled for the first time the AngII/Rac1/STAT3 signaling as a mechanism for ASR thereby AF in a particular clinical setting―RHD patients with persistent AF and indicated inhibition of this pathway may help ameliorating adverse ASR.





Similar content being viewed by others
References
Benjamin, E. J., Wolf, P. A., D’Agostino, R. B., Silbershatz, H., Kannel, W. B., & Levy, D. (1998). Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation, 98, 946–952.
Nattel, S. (2002). New ideas about atrial fibrillation 50 years on. Nature, 415, 219–226.
Haïssaguerre, M., Jaïs, P., Shah, D. C., Takahashi, A., Hocini, M., Quiniou, G., et al. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New England Journal of Medicine, 339, 659–666.
Hwang, C., Wu, T. J., Doshi, R. N., Peter, C. T., & Chen, P. S. (2000). Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation, 101, 1503–1505.
Allessie, M., Ausma, J., & Schotten, U. (2002). Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovascular Research, 54, 230–246.
Diker, E., Aydogdu, S., Ozdemir, M., Kural, T., Polat, K., Cehreli, S., et al. (1996). Prevalence and predictors of atrial fibrillation in rheumatic valvular heart disease. American Journal of Cardiology, 77, 96–98.
Cardin, S., Libby, E., Pelletier, P., Le Bouter, S., Shiroshita-Takeshita, A., Le Meur, N., et al. (2007). Contrasting gene expression profiles in two canine models of atrial fibrillation. Circulation Research, 100, 425–433.
Everett, T. H., Li, H., Mangrum, J. M., McRury, I. D., Mitchell, M. A., Redick, J. A., et al. (2000). Electrical, morphological, and ultrastructurat remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation, 102, 1454–1460.
Li, D., Shinagawa, K., Pang, L., Leung, T. K., Cardin, S., Wang, Z., et al. (2001). Effects of angiotensin converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation, 104, 2608–2614.
Nazir, S. A., & Lab, M. J. (1996). Mechanoelectric feedback in the atrium of the isolated guinea-pig heart. Cardiovascular Research, 32, 112–119.
Sánchez-Quintana, D., López-Mínguez, J. R., Pizarro, G., Murillo, M., & Cabrera, J. A. (2012). Triggers and anatomical substrates in the genesis and perpetuation of atrial fibrillation. Current Cardiology Reviews, 8, 310–326.
Duffy, H. S., & Wit, A. L. (2008). Is there a role for remodeled connexins in AF? No simple answers. Journal of Molecular and Cellular Cardiology, 44, 4–13.
van der Velden, H. M., Ausma, J., Rook, M. B., Hellemons, A. J., van Veen, T. A., Allessie, M. A., et al. (2000). Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovascular Research, 46, 476–486.
Thijssen, V. L., Ausma, J., & Borgers, M. (2001). Structural remodelling during chronic atrial fibrillation: Act of programmed cell survival. Cardiovascular Research, 52, 14–24.
Kostin, S., Klein, G., Szalay, Z., Hein, S., Bauer, E. P., & Schaper, J. (2002). Structural correlate of atrial fibrillation in human patients. Cardiovascular Research, 54, 361–379.
Climent, V., Hurlé, A., Ho, S. Y., Sáenz-Santamaría, J., Nogales, A. G., & Sánchez-Quintana, D. (2004). Early morphologic changes following microwave endocardial ablation for treatment of chronic atrial fibrillation during mitral valve surgery. Journal of Cardiovascular Electrophysiology, 5, 1277–1283.
Ausma, J., Wijffels, M., Thoné, F., Wouters, L., Allessie, M., & Borgers, M. (1997). Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation, 96, 3157–3163.
Spach, M. S. (2007). Mounting evidence that fibrosis generates a major mechanism for atrial fibrillation. Circulation Research, 101, 743–745.
Ausma, J., Wijffels, M., Thoné, F., Wouters, L., Allessie, M., & Borgers, M. (2008). Angiotensin II activates signal transducer and activators of transcription 3 via Rac1 in atrial myocytes and fibroblasts: Implication for the therapeutic effect of statin in atrial structural remodeling. Circulation, 117, 344–355.
Fukui, A., Takahashi, N., Nakada, C., Masaki, T., Kume, O., Shinohara, T., et al. (2013). Role of leptin signaling in the pathogenesis of angiotensin II-mediated atrial fibrosis and fibrillation. Circulation, 6, 402–409.
Ehrlich, J. R., Hohnloser, S. H., & Nattel, S. (2006). Role of angiotensin system and effects of its inhibition in atrial fibrillation: Clinical and experimental evidence. European Heart Journal, 27, 512–518.
Sun, Y., Ramires, F. J., & Weber, K. T. (1997). Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovascular Research, 35, 138–147.
Gu, J., Liu, X., Wang, Q. X., Tan, H. W., Guo, M., Jiang, W. F., et al. (2012). Angiotensin II increases CTGF expression via MAPKs/TGF-β1/TRAF6 pathway in atrial fibroblasts. Experimental Cell Research, 318, 2105–2115.
Boldt, A., Wetzel, U., Weigl, J., Garbade, J., Lauschke, J., Hindricks, G., et al. (2003). Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease. Journal of the American College of Cardiology, 42, 1785–1792.
Reil, J. C., Hohl, M., Oberhofer, M., Kazakov, A., Kaestner, L., Mueller, P., et al. (2010). Cardiac Rac1 overexpression in mice creates a substrate for atrial arrhythmias characterized by structural remodelling. Cardiovascular Research, 87, 485–493.
Adam, O., Frost, G., Custodis, F., Sussman, M. A., Schäfers, H. J., Böhm, M., et al. (2007). Role of Rac1 GTPase activation in atrial fibrillation. Journal of the American College of Cardiology, 50, 359–367.
Adam, O., Lavall, D., Theobald, K., Hohl, M., Grube, M., Ameling, S., et al. (2010). Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. Journal of the American College of Cardiology, 55, 469–480.
Tsai, C. T., Lin, J. L., Lai, L. P., Lin, C. S., & Huang, S. K. (2008). Membrane translocation of small GTPase Rac1 and activation of STAT1 and STAT3 in pacing-induced sustained atrial fibrillation. Heart Rhythm, 5, 1285–1293.
Gupta, D. K., Shah, A. M., Giugliano, R. P., Ruff, C. T., Antman, E. M., Grip, L. T., Deenadayalu, N., Hoffman, E., Patel, I., Shi, M., Mercuri, M., Mitrovic, V., Braunwald, E., Solomon, S. D. (2013). Effective aNticoaGulation with factor xA next GEneration in AF-thrombolysis in myocardial infarction 48 (ENGAGE AF-TIMI 48) Echocardiographic Study Investigators. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. European Heart Journal, 35(22), 1457–1465.
De Sisti, A., Leclercq, J. F., Halimi, F., Fiorello, P., Bertrand, C., & Attuel, P. (2014). Evaluation of time course and predicting factors of progression of paroxysmal or persistent atrial fibrillation to permanent atrial fibrillation. Pacing and Clinical Electrophysiology, 37(3), 345–355.
Yoshida, K., Rabbani, A. B., Oral, H., Bach, D., Morady, F., & Chugh, A. (2011). Left atrial volume and dominant frequency of atrial fibrillation in patients undergoing catheter ablation of persistent atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 32, 155–161.
Wozakowska-Kapłon, B. (2005). Changes in left atrial size in patients with persistent atrial fibrillation: A prospective echocardiographic study with a 5-year follow-up period. International Journal of Cardiology, 101, 47–52.
Dostal, D. E., Hunt, R. A., Kule, C. E., Bhat, G. J., Karoor, V., McWhinney, C. D., et al. (1997). Molecular mechanisms of angiotensin II in modulating cardiac function: Intracardiac effects and signal transduction pathways. Journal of Molecular and Cellular Cardiology, 29, 2893–2902.
Sugden, P. H., & Clerk, A. (1997). Regulation of the ERK subgroup of MAP kinase cascades through G protein-coupled receptors. Cellular Signalling, 9, 337–351.
Ushio-Fukai, M., Alexander, R. W., Akers, M., & Griendling, K. K. (1998). Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. The Journal of Biological Chemistry, 73, 15022–15029.
Zou, Y., Komuro, I., Yamazaki, T., Kudoh, S., Aikawa, R., Zhu, W., et al. (1998). Cell type-specific angiotensin II-evoked signal transduction pathways: Critical roles of Gbetagamma subunit, Src family, and Ras in cardiac fibroblasts. Circulation Research, 82, 337–345.
Brown, J. L., Stowers, L., Baer, M., Trejo, J., Coughlin, S., & Chant, J. (1997). Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Current Biology, 6, 598–605.
Abo, A., Pick, E., Hall, A., Totty, N., Teahan, C. G., & Segal, A. W. (1991). Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature, 353, 668–670.
Dudley, S. C, Jr, Hoch, N. E., McCann, L. A., Honeycutt, C., Diamandopoulos, L., Fukai, T., et al. (2005). Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: Role of the NADPH and xanthine oxidases. Circulation, 112, 1266–1273.
Takemoto, M., Node, K., Nakagami, H., Liao, Y., Grimm, M., Takemoto, Y., et al. (2001). Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. The Journal of Clinical Investigation, 108, 1429–1437.
Adam, O., Neuberger, H. R., Böhm, M., & Laufs, U. (2008). Prevention of atrial fibrillation with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Circulation, 118, 1285–1293.
Yue, H., Li, W., Desnoyer, R., & Karnik, S. S. (2010). Role of nuclear unphosphorylated STAT3 in angiotensin II type 1 receptor-induced cardiac hypertrophy. Cardiovascular Research, 85, 90–99.
Paradis, P., Dali-Youcef, N., Paradis, F. W., Thibault, G., & Nemer, M. (2000). Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proceedings of the National Academy of Sciences of the United States of America, 97, 931–936.
Conflict of interest
The authors have not declared any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, XD., Huang, JH. & Wang, HS. Angiotensin II Activates Signal Transducers and Activators of Transcription 3 via Rac1 in the Atrial Tissue in Permanent Atrial Fibrillation Patients with Rheumatic Heart Disease. Cell Biochem Biophys 71, 205–213 (2015). https://doi.org/10.1007/s12013-014-0186-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0186-z