Abstract
The cure rates are much lower for multidrug-resistant (MDR) tuberculosis (TB) patients. Delamanid (OPC-67683) has been evaluated in phase-II MDR-TB clinical trials. Herein, we reviewed MDR-TB cases in which treatment regimens, with/without delamanid, were administered. Thirty-eight patients were enrolled; 26 received delamanid-containing regimens (treatment group) while 12 received placebo-containing regimens (control group) for 56 days. Data regarding clinical/radio-microbiological characteristics, drug tolerability, and treatment outcomes were collected. We found that all patients had isolates resistant to a median of 5 (range 2–7) drugs; 24 (92.3 %) patients in treatment group and 11 (91.7 %) in control group had cavities. Culture conversion was obtained in 32 pulmonary TB cases (median 74.5 days). At data censure, 30/38 patients successfully completed therapy with documented negative cultures for at least 18 months before the end of treatment. Two patients (5 consecutive negative cultures) are still on treatment. Six patients had poor outcome (3 failures/2 lost/1 death). In 13 patients, adverse events were observed that included mental disorder, QT interval prolongation, and increased blood cortisol whereas only 3 patients stopped delamanid treatment because of adverse events. It was, therefore, concluded that delamanid was well-tolerated, had low rates of discontinuation, and could be effective for treating MDR-TB.
Similar content being viewed by others
Introduction
Tuberculosis (TB) treatment has become very difficult after the emergence of multidrug-resistant (MDR) TB, which refers to Mycobacterium tuberculosis isolates resistant to at least isoniazid and rifampicin, the two most powerful anti-TB drugs. Extensively drug resistant (XDR) TB refers to MDR-TB strains that are also resistant to any fluoroquinolone and at least one second-line injectable (kanamycin, amikacin, or capreomycin) [1–4]. China is one of the 22 countries of the world with the highest burden of tuberculosis [5]. China is one of the 27 countries in the world that has the highest number of MDR-TB/XDR-TB cases [6]. Due to the extent of drug resistance, treatment options for MDR-TB and XDR-TB are limited and, therefore, effective drugs are highly needed [7, 8].
Delamanid (OPC-67683), a nitro-dihydro-imidazooxazole derivative, is a mycolic acid biosynthesis inhibitor. This drug is reported to be free of mutagenicity and possesses highly potent activity against TB including MDR-TB as shown by its exceptionally low minimum inhibitory concentration (MIC) (range: 0.006–0.024 μg/ml) in vitro and highly effective therapeutic activity at low doses in vivo [9–12]. Its activity at a concentration of 0.1 μg/ml was reported to be similar to that of the first-line drug rifampicin at a concentration of 3 μg/ml. Besides, delamanid shows no cross-resistance with any of the currently used anti-TB drugs [11]. In the present study, we analyzed tolerance and safety of delamanid as well as treatment outcomes among a cohort of 38 MDR-/XDR-TB patients who were treated with or without delamanid as a part of MDR-TB treatment regimens in Shanghai Pulmonary Hospital, Shanghai, China. Herein, we report that delamanid is well-tolerated, has low rates of discontinuation, and can be effective for treating MDR-TB.
Patients and Methods
Study Population
Patients were recruited from Shanghai Pulmonary Hospital in Shanghai, China which is one of the sites of two consecutive global multi-center clinical trials entitled: (1) “A multi-center, randomized, double-blinded, placebo-controlled phase-II trial to evaluate safety, efficacy, and pharmacokinetics of multiple doses of delamanid in patients with pulmonary sputum culture-positive, MDR tuberculosis” (Trial 204) [13]; (2) “A phase-II, multi-center, uncontrolled, open-label trial to evaluate safety, tolerability, and efficacy of orally administered delamanid as 100 mg BID with optional titration to 20 mg BID for up to 6 months exposure in patients with pulmonary MDR tuberculosis” (Trial 208). These two trials were sponsored by Otsuka Pharmaceutical Development and Commercialization, Japan. The patients with culture-positive MDR pulmonary tuberculosis were screened regarding eligibility for enrolment in the study. The inclusion criteria were as follows: (1) written, informed consent; (2) male or female aged 18–64 years; (3) either mycobacterial culture of sputum positive for growth or sputum smear positive for acid-fast bacilli with a positive rapid test for rifampicin resistance on direct sputum, 60 days before the expected date of enrollment; (4) patients with TB caused by isolates of M. tuberculosis complex confirmed to be resistant to isoniazid and rifampicin. The exclusion criteria were as follows: (1) a history of allergy to any nitro-imidazole derivatives; (2) clinically relevant changes in the echocardiography (ECG), such as prolongation of either QTcF or QTcB interval over 430 ms in male patients and 450 ms in female patients. The patients who completed Trial 204 could be rolled over to Trial 208. The two studies were approved by the Shanghai Pulmonary Hospital Ethics Committee and written informed consent was obtained from all patients before enrolment.
Randomization and Masking
Patients were randomly allocated to receive either delamanid (Otsuka Pharmaceutical Development and Commercialization, Japan) or organoleptically identical placebo (Otsuka Pharmaceutical Development and Commercialization, Japan) with a 2:1 ratio. Before the start of recruitment, a randomization sequence had been prepared by the sponsor. On recruitment, investigators enrolling patients evaluated if cavity was present or not according to the patient’s baseline chest radiograph. Those without cavity were allocated consecutive randomization numbers from 2000 to 2999, and those with cavity were allocated consecutive randomization numbers from 1000 to 1999. Both investigators and patients did not know the active drug or placebo in Trial 204, while those analyzing the data knew the blind code.
Procedures
The first dose of investigational medicinal product (IMP) was administered within 7 days of the start of anti-TB optimal background regimen (OBR) treatment. IMP was given for 56 days in Trial 204. Patients were then observed for 28 days. All patients received OBR in addition to the IMP for at least 24 months continuously. OBR was tailored according to the susceptibility results of each isolate and following the guidelines of WHO emergency update 2008 [14]. The baseline clinical assessment consisted of chest radiography, measurement of height and weight, and collection of a sputum sample (for microscopy and culture). Patients were hospitalized for 56 days after starting the IMP and were reviewed at 63, 70, 77, 84 days. At each time point, a sputum specimen was collected from all patients, physical examination was performed, vital signs were measured as well as urine and blood samples were collected and analyzed. Chest radiography was repeated for 56 days. The patients who completed the treatment with IMP and were observed for at least 28 days and had also signed the informed consent for Trial 208 could roll over to Trial 208 in which they were all treated openly with delamanid for 6 months as well as OBR. Directly observed therapy (DOT) was given throughout the treatment. Treatment duration varied but with a minimum of 24 months. Treatment regimens included delamanid (or placebo during Trial 204) and at least four drugs selected according to past treatment and recent susceptibility results. Delamanid was administered orally at a dosage of 200–400 mg daily. Sputum culture was performed using liquid media-mycobacteria growth indicator tube (MGIT) and solid agar media. Drug susceptibility testing was done by MGIT and solid media impregnated by anti-TB drugs. The laboratory performing drug susceptibility testing was quality controlled within the WHO standard program.
We recorded individual case data at Shanghai Pulmonary Hospital. Data included patients’ demographic characteristics and comorbid conditions; clinical, radiographic, and microbiological characteristics; serial sputum smear and culture results; treatment regimens, duration, outcomes; and drug toxicity and tolerability. We evaluated the treatment outcome of IMP-containing regimens by clinical and sputum culture conversion. Safety and tolerability of delamanid were evaluated by reviewing blood and urine tests and by assessing symptoms record each month.
Standard definitions were used for MDR-TB, i.e., tuberculosis with documented resistance to at least isoniazid and rifampicin as well as for treatment outcomes [15]. Cure was defined by at least five consecutive negative cultures from samples collected at least 30 days apart in the final 12 months and no positive culture during the last 18 months of treatment, including an end-of-treatment specimen. Treatment completed referred to the patients who had completed treatment according to protocol but did not meet the definition for cure because of lack of bacteriological results. Death was defined as a patient who died for any reason during the course of MDR-TB treatment. Treatment failure was defined as two or more of the five cultures recorded in the final 12 months of therapy being positive, or if any of the final three cultures were positive. Default was defined as patients whose treatment was interrupted for two or more consecutive months for any reason without medical approval. An unsatisfactory outcome was defined as default, treatment failure, or death.
Statistical Analysis
Data were analyzed using SPSS 15.0 statistical software. The Pearson Chi square test was used to analyze proportions. All P values < 0.05 were considered statistically significant.
Results
Demographic Characteristics
From October 2008 to October 2010, 38 cases of MDR-TB were enrolled in Trial 204. Twenty-six of 38 patients were treated with delamanid as part of a multidrug regimen for MDR-TB. The remaining 12 patients were treated with placebo as part of a regimen for MDR-TB. All patients were Chinese, including 29 males and 9 females, aged 19–56 years with the mean of 34.8 ± 11.1 years (summarized in Table 1). All patients were human immunodeficiency virus (HIV) status negative.
Disease Status
Thirty-eight patients had a mean history of 19.4 months of previous treatment for tuberculosis. Except for pulmonary tuberculosis, 4 of the 38 (10.5 %) patients had endobronchial TB, 1 (2.6 %) had tuberculous meningitis, 1 (2.6 %) had tuberculous empyema, and 5 (13.2 %) had diabetes mellitus. Thirty-five (92.1 %) patients had cavitation observed on baseline chest radiograph or chest computed tomography, and all 38 (100 %) patients had positive sputum-culture microscopy results at the time of MDR-TB diagnosis. The median number of drugs to which 38 culture-positive isolates were found resistant was 5 (range: 2–7 drugs). Drug resistance profile of each patient is listed in Table 1.
Treatment
The median number of drugs given for MDR-TB treatment was 6 and each regimen included a fluoroquinolone and an injectable agent unless resistance was noted to these classes of drugs. The mean duration of delamanid administration was 200 ± 62 (range: 4–238) days. Table 1 shows general characteristics in each case. Thirty-four of the 38 patients rolled over to Trial 208 after they had finished with Trial 204; however, 3 patients were not recruited to Trial 208 by doctors because of the adverse events. One patient refused to enroll.
Treatment Outcomes
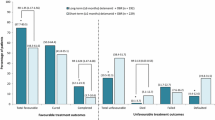
Therapy outcome data are summarized in Table 1. At the time of completion of Trial 204 on day 84, regarding treatment group, 18 of 26 (69.2 %) patients got sputum culture conversion; while in control (placebo) group, 5 of 12 patients (41.7 %) got sputum culture conversion (χ 2 = 2.622; P = 0.106). One culture of the above 18 patients in treatment group became positive again in Trial 208. After Trial 208 was completed, at data censure (Dec 30, 2011), there was one death and three failures recorded. Two patients were lost to follow up (withdrew consent) during Trial 208. Thirty out of 38 (78.9 %) patients had been cured or completed treatment. Two patients (5.3 %) were still receiving treatment. These 32 of the 38 (84.2 %) patients got at least five consecutive negative cultures from sputum collected at least 30 days apart. The median time to culture conversion was 74.5 days after the beginning of MDR-TB therapy (range: 15–436 days). Culture results were occasionally positive in three patients (7.9 %).
Treatment Toxicity
Regarding Trial 204, the incidence rates of adverse events in treatment and control groups were 76.9 % (20/26) and 75 % (9/12), respectively. There was no significant difference found between two groups (χ 2 = 0.017, P = 0.897). The main adverse events unrelated to delamanid included insomnia, ear noise, skin rash, and leukopenia. Two patients in treatment group stopped delamanid therapy due to mental disorder, likely attributable to the combination of isoniazid, levofloxacin and delamanid. One patient presented serious complaint and was recovered after treatment of narcotics (anxiolytic). The other case was mild and recovered without specific medication. Delamanid therapy was not restarted for these two patients. One patient in treatment group developed mild QT interval prolongation attributable to delamanid. The prolongation did not exceed 500 ms and the patient experienced no clinical manifestations. Delamanid treatment was continued and no other intervention was undertaken. Twenty-three (88.5 %) cases reported no toxicity likely attributable to the delamanid therapy.
Regarding Trial 208, morning blood cortisol level was elevated in 10 patients, generally within only onefold above normal range, which was probably related to delamanid (no patient was above the normal range at the beginning of the trial), but the therapy was not discontinued. These patients presented no symptoms and hence no special treatment was instituted. High-level blood cortisol in nine patients receded to normal level after the completion of Trial 208. For the 10th patient, it still remained above normal levels and the patient was kept under close observation. One patient withdrew from Trial 208 because QTcF at baseline did not meet the criteria for entering the trial, the patient experienced no clinical manifestations from the increase. Treatment toxicity data for all patients are summarized in Table 2.
Discussion
This study represents the first report with final outcomes regarding use of delamanid-containing regimens for the treatment of MDR-TB. Herein, we present the results obtained from 38 patients receiving delamanid therapy (at a dosage of 100 mg twice daily or 200 mg twice daily) or placebo. Compared to patients with susceptible tuberculosis, patients with MDR-TB have terribly unfavorable short- and long-term outcomes because of the lack of potent anti-TB drugs, the longer treatment duration of 24–30 months, and the side effects/toxicities of second-line medications which often induce nonadherence to treatment [16, 17]. The range of treatment success for MDR-TB reported in the literature is quite varied; two large meta-analyses of MDR-TB treatment cohorts including thousands of patients have reported an overall success in the range is 55–65 % [18, 19]. In the present study, the median number of drugs given for MDR-TB treatment was 6, and each regimen included a fluoroquinolone and a second-line injectable agent. Of note, the recommended regimen for MDR by WHO includes a combination of at least four drugs effective for M. tuberculosis isolates [14]. We chose these drugs following a stepwise selection process through five groups [14] based on their efficacy, safety, and cost. Furthermore, it was suggested that at least five different medications should be used in order to optimize the chance for a successful treatment outcome [20–23]. The efficacy data from our Trial 204 showed that delamanid, given at the rate of 100 mg or 200 mg twice daily plus OBR group achieved 69.2 % sputum culture conversion based on culture in MGIT system at 3 months. In contrast, 41.7 % achieved sputum culture conversion at 3 months in the placebo plus OBR group; however, the difference was statistically nonsignificant. At data censure, 30 of the 38 (78.9 %) patients had successfully completed therapy with documented negative cultures for at least 18 months before the end of treatment. As MDR-TB patients are very heterogeneous as a group, long-term efficacy data collected from the 481 patients enrolled globally in Trial 204 [13] would be beneficial to determine if the encouraging results that we obtained in the Chinese patient population could be applied more broadly to the entire population of MDR-TB trials.
In our patient population, only minor side effects and toxicities likely attributable or attributable to delamanid were observed which included mental disorder, increased blood cortisol, and QT interval prolongation. Two patients developed mental disorder likely related to the combination of isoniazid, levofloxacin and delamanid and withdrew from the trial. They were simultaneously treated with high-dose isoniazid (15 mg/kg/day) and levofloxacin (Lfx) as part of OBR. One individual presented serious paranoid state as treated with delamanid at the dose rate of 200 mg twice daily. He recovered after delamanid, isoniazid, and levofloxacin were discontinued and treated with anxiolytic drugs. The other person expressed mild dysphoria when treated with delamanid at the dose rate of 100 mg twice daily and recovered after delamanid, isoniazid, and levofloxacin were discontinued with no other salvage therapy given. We speculate that this toxicity might have been induced by the combination of the above three drugs since mental disorder can also be related to isoniazid and Lfx. Notably, although most of other patients in this study were also treated with the combination of above three drugs, they did not show similar side effects. In contrast, for the overall population of 481 patients enrolled worldwide in Trial 204, the only psychiatric adverse event that was proportionately different between the two treatment groups and the placebo group was depression at 3.1 and 2.5 versus 8.1 %, respectively; this difference was significantly higher for the patients receiving placebo (P = 0.0437) [13]. In addition, none of the other sites outside of China used high-dose isoniazid. In regard with blood cortisol, the proportion was 29.4 % (10/34) and delamanid was associated with a mean increase of onefold above the normal range compared to baseline, while patients had clinically apparent evidence of elevated cortisol. By comparison for the overall trial, 2.5 % of patients in 100 mg twice daily group and 3.1 % of patients in 200 mg twice daily group experienced increased blood cortisol levels versus 0.6 % of placebo patients; however, the difference was nonsignificant (P = 0.1711) [13]. Besides, QT interval prolongation was observed in 2 patients but without clinical symptoms. QT interval prolongation of one patient treated by delamanid 200 mg twice daily in Trial 204 and 100 mg daily in Trial 208 was mild and he completed Trails 204 and 208. The other patient treated by delamanid 100 mg twice daily was excluded from Trial 208, because the QTcF at baseline did not meet the criteria for entering the trial. These findings warrant caution in patients with underlying cardiac disease or electrolyte abnormalities when given delamanid with other drugs that prolong QT interval duration. However, close monitoring of side effects or toxicities with expert consultation, rather than immediate withdrawal of delamanid may prove beneficial since most patients in this study did not develop serious or worsening toxicities attributable to delamanid. Of note, this is an important point to emphasize since there are very few effective medications available for the treatment of MDR-TB. Dealing with possible side effects due to other anti-TB drugs while adhering to the treatment regimen is critical to successful treatment outcome in difficult cases. All patients in this study were either hospitalized or received DOT during the treatment duration up until completion. DOT is important and DOT throughout the whole course of treatment is essential for achieving favorable treatment outcomes and adherence for treating MDR-TB [24]. Assessment of the long-term clinical data from larger local and international clinical trials would still be required to evaluate efficacy profile of this drug.
In conclusion, addition of delamanid to the available treatment regimen for MDR-TB may be clinically beneficial. As a part of MDR-TB regimen, it has good efficacy with acceptable toxicity. Importantly, while receiving delamanid, patients should be closely monitored for signs or symptoms of mental illness, especially when patients receive delamanid in combination with high-dose isoniazid and fluoroquinolones. Contraindications include the cardiac disorders and other drugs that prolong QT interval duration. Therefore, ECG should be performed before starting therapy and then done at least 6 weeks after treatment initiation by which time point patients have reached steady state for Delamanid and all relevant metabolites. Patients with previous mental and/or cardiac disease may not be the suitable candidates to receive this drug. Further randomized clinical trials of delamanid treatment should help improve clinical benefit(s) in MDR-TB patients.
References
Wright, A., Zignol, M., Van Deun, A., et al. (2009). Epidemiology of antituberculosis drug resistance 2002–07: An updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet, 373, 1861–1873.
Kim, D. H., Kim, H. J., Park, S.-K., et al. (2008). Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. American Journal of Respiratory and Critical Care Medicine, 178, 1075–1082.
Kwon, Y. S., Kim, Y. H., Suh, G. Y., et al. (2008). Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clinical Infectious Diseases, 47, 496–502.
Mitnick, C., Castro, K., Harrington, M., Sacks, L., & Burman, W. (2007). Randomized trials to optimize treatment of multidrug-resistant tuberculosis. PLoS Medicine, 4, e292.
World Health Organization. (2008). Global tuberculosis control: Surveillance, planning, financing. WHO report 2008. WHO/HTM/TB/2008.393. Geneva, Switzerland.
World Health Organization. (2010). Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO/HTM/TB/2010.3.
Stop TB Partnership and World Health Organization. (2006). Global plan to stop TB 2006–2015. WHO/HTM/STB/2006.35 Geneva: World Health Organization.
World Health Organization. (2006). Addressing the threat of tuberculosis caused by extensively drug-resistant Mycobacterium tuberculosis. Weekly Epidemiology Record, 81, 386–390.
Matsumoto, M., Hiroyuki, H., Tatsuo, T., et al. (2006). OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. Public Library Science Medicine, 3, e466.
Matsumoto M. (2003). In vitro activities of OPC-67683 and reference compounds against Mycobacterium tuberculosis standard strains and Mycobacterium bovis strains. Otsuka Study No. 019064.
Matsumoto M. (2003). In vitro activities of OPC-67683 and reference compounds against clinically isolated Mycobacterium tuberculosis. Otsuka Study No. 019196, Otsuka Report No. 019196, Otsuka Report No. 015366.
Matsumoto M. (2005). Inhibitory activity of OPC-67683 against mycolic acid synthesis in Mycobacterium bovis BCG. Otsuka Study No. 021327, Otsuka Report No. 017300.
Gler, M. T., Skripconoka, V., Sanchez-Garavito, E., et al. (2012). Delamanid for patients with multi-drug resistant pulmonary tuberculosis. The New England Journal of Medicine, 366, 1–10.
World Health Organization (2008). Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: Emergency Update 2008. WHO/HTM/TB/2008.402.
World Health Organization. (2010). Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO/HTM/TB/2010.3.
Schecter, G. F., Scott, C., True, L., Raftery, A., Flood, J., & Mase, S. (2010). Linezolid in the treatment of multidrug-resistant tuberculosis. Clinical Infectious Diseases, 50, 49–55.
Chan, E., & Iseman, M. (2008). Multidrug-resistant and extensively drug-resistant tuberculosis: A review. Current Opinion in Infectious Diseases, 21, 587–595.
Orenstein, E. W., Basu, S., Shah, N. S., et al. (2009). Treatment outcomes among patients with multidrug-resistant tuberculosis: Systematic review and meta-analysis. Lancet Infectious Diseases, 9, 153–161.
Johnston, J. C., Shahidi, N. C., Sadatsafavi, M., et al. (2009). Treatment outcomes of multidrug-resistant tuberculosis: A systematic review and meta-analysis. PLoS ONE, 4(9), e6914.
Mitnick, C., Bayona, J., Palacios, E., et al. (2003). Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. The New England Journal of Medicine, 348, 119–128.
Leimane, V., Reikstina, V., Holtz, T., et al. (2005). Clinical outcomes of individualized treatment of multidrug-resistant tuberculosis in Latvia: A retrospective cohort study. Lancet, 365, 318–326.
Caminaro, J. A. (2006). Treatment of multidrug-resistant tuberculosis: Evidence and controversies. International Journal of Tuberculosis and Lung Diseases, 10, 829–837.
Chan, E. D., Laurel, V., Strand, M. J., et al. (2004). Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. American Journal of Critical Care Medicine, 169, 1103–1109.
WHO. (2006). Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: WHO.
Acknowledgments
The authors thank all the participants at Shanghai Pulmonary Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qing Zhang and Yidian Liu contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhang, Q., Liu, Y., Tang, S. et al. Clinical Benefit of Delamanid (OPC-67683) in the Treatment of Multidrug-Resistant Tuberculosis Patients in China. Cell Biochem Biophys 67, 957–963 (2013). https://doi.org/10.1007/s12013-013-9589-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9589-5