Abstract
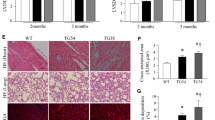
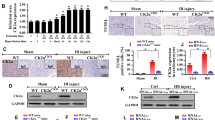
Copper metabolism MURR domain 1 (COMMD1) increases in ischemic myocardium along with suppressed contractility. Cardiomyocyte-specific deletion of COMMD1 preserved myocardial contractile function in response to the same ischemic insult. This study was undertaken to test the hypothesis that cardiomyocyte protection in COMMD1 myocardium is responsible for the functional preservation of the heart in response to ischemic insult. After ischemic insult, there were significantly more cardiomyocytes in the cardiomyocyte-specific COMMD1 deletion myocardium than that in WT controls. This preservation of cardiomyocytes was paralleled by a significant suppression of apoptosis in the COMMD1 deletion myocardium compared to controls. In searching for the mechanistic understanding of the anti-apoptotic effect of COMMD1 deletion, we found the anti-apoptotic Bcl-2 mRNA and protein expression were upregulated and the pro-apoptotic Bax mRNA and protein expression were downregulated. The critical transcription factor RelA, maintaining a high ratio between Bcl-2 and Bax for anti-apoptotic action, was suppressed by ischemia, but was rescued in the COMMD1 deletion myocardium. Because COMMD1 is critically involved in RelA ubiquitination and degradation, the data obtained here demonstrate that COMMD1 deletion leads to RelA preservation in ischemic myocardium, promoting the Bcl-2 anti-apoptotic pathway and suppressing the Bax pro-apoptotic pathway, and in combination, leading to protection of cardiomyocytes from ischemia-induced apoptosis.




Similar content being viewed by others
References
Materia, S., Cater, M. A., Klomp, L. W., Mercer, J. F., & La Fontaine, S. (2012). Clusterin and COMMD1 independently regulate degradation of the mammalian copper ATPases ATP7A and ATP7B. The Journal of biological chemistry, 287, 2485–2499.
Phillips-Krawczak, C. A., Singla, A., Starokadomskyy, P., Deng, Z., Osborne, D. G., Li, H., Dick, C. J., Gomez, T. S., Koenecke, M., Zhang, J. S., Dai, H., Sifuentes-Dominguez, L. F., Geng, L. N., Kaufmann, S. H., Hein, M. Y., Wallis, M., McGaughran, J., Gecz, J., Sluis, B. V., … Burstein, E. (2015). COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Molecular biology of the cell, 26, 91–103.
Campbell, K. J., & Perkins, N. D. (2006). Regulation of NF-kappaB function. Biochemical Society symposium, 165–180.
Thoms, H. C., Loveridge, C. J., Simpson, J., Clipson, A., Reinhardt, K., Dunlop, M. G., & Stark, L. A. (2010). Nucleolar targeting of RelA(p65) is regulated by COMMD1-dependent ubiquitination. Cancer research, 70, 139–149.
Bartuzi, P., Hofker, M. H., & van de Sluis, B. (2013). Tuning NF-κB activity: a touch of COMMD proteins. Biochimica et biophysica acta, 1832, 2315–2321.
van de Sluis, B., Mao, X., Zhai, Y., Groot, A. J., Vermeulen, J. F., van der Wall, E., van Diest, P. J., Hofker, M. H., Wijmenga, C., Klomp, L. W., Cho, K. R., Fearon, E. R., Vooijs, M., & Burstein, E. (2010). COMMD1 disrupts HIF-1alpha/beta dimerization and inhibits human tumor cell invasion. The Journal of clinical investigation, 120, 2119–2130.
Vonk, W. I., Wijmenga, C., Berger, R., van de Sluis, B., & Klomp, L. W. (2010). Cu, Zn superoxide dismutase maturation and activity are regulated by COMMD1. The Journal of biological chemistry, 285, 28991–29000.
Li, K., Li, C., Xiao, Y., Wang, T., & James Kang, Y. (2018). The loss of copper is associated with the increase in copper metabolism MURR domain 1 in ischemic hearts of mice. Experimental biology and medicine (Maywood, N.J.), 243, 780–785.
Li, C., Wang, T., Xiao, Y., Li, K., Meng, X., & James, Kang Y. (2021). COMMD1 upregulation is involved in copper efflux from ischemic hearts. Experimental biology and medicine (Maywood, N.J.), 246, 607–616.
Zhang, J., Qiu, W., Ma, J., Wang, Y., Hu, Z., Long, K., Wang, X., Jin, L., Tang, Q., Tang, G., Zhu, L., Li, X., Shuai, S., & Li, M. (2019). miR-27a-5p Attenuates Hypoxia-induced Rat Cardiomyocyte Injury by Inhibiting Atg7. International journal of molecular sciences, 20, 2418.
Haunstetter, A., & Izumo, S. (2000). Toward antiapoptosis as a new treatment modality. Circulation research, 86, 371–376.
Yaoita, H., Ogawa, K., Maehara, K., & Maruyama, Y. (1998). Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation, 97, 276–281.
Balsam, L. B., Kofidis, T., & Robbins, R. C. (2005). Caspase-3 inhibition preserves myocardial geometry and long-term function after infarction. The Journal of surgical research, 124, 194–200.
Kajstura, J., Cheng, W., Reiss, K., Clark, W. A., Sonnenblick, E. H., Krajewski, S., Reed, J. C., Olivetti, G., & Anversa, P. (1996). Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Laboratory investigation; a journal of technical methods and pathology, 74, 86–107.
Takashi, E., & Ashraf, M. (2000). Pathologic assessment of myocardial cell necrosis and apoptosis after ischemia and reperfusion with molecular and morphological markers. Journal of molecular and cellular cardiology, 32, 209–224.
Webster, K. A. (2012). Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future cardiology, 8, 863–884.
Liang, H., Yang, C. X., Zhang, B., Wang, H. B., Liu, H. Z., Lai, X. H., Liao, M. J., & Zhang, T. (2015). Sevoflurane suppresses hypoxia-induced growth and metastasis of lung cancer cells via inhibiting hypoxia-inducible factor-1α. Journal of anesthesia, 29, 821–830.
Schimmer, A. D., Dalili, S., Batey, R. A., & Riedl, S. J. (2006). Targeting XIAP for the treatment of malignancy. Cell death and differentiation, 13(2), 179–188.
Galbán, S., & Duckett, C. S. (2010). XIAP as a ubiquitin ligase in cellular signaling. Cell death and differentiation, 17, 54–60.
Jiang, L., Ning, Z., & Hong, T. (2005). The role of bc1-2 and bax as well as NF-kB on liver cancer cell apoptosis induced by resveratrol. Chinese Journal of Microecology, 17, 164–165.
Kucharczak, J., Simmons, M. J., Fan, Y., & Gélinas, C. (2003). To be, or not to be: NF-kappaB is the answer–role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene, 22, 8961–8982.
Maine, G. N., Mao, X., Komarck, C. M., & Burstein, E. (2007). COMMD1 promotes the ubiquitination of NF-kappaB subunits through a cullin-containing ubiquitin ligase. The EMBO journal, 26, 436–447.
Geng, H., Wittwer, T., Dittrich-Breiholz, O., Kracht, M., & Schmitz, M. L. (2009). Phosphorylation of NF-kappaB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO reports, 10, 381–386.
Beg, A. A., Sha, W. C., Bronson, R. T., Ghosh, S., & Baltimore, D. (1995). Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature, 376, 167–170.
Panta, G. R., Kaur, S., Cavin, L. G., Cortés, M. L., Mercurio, F., Lothstein, L., Sweatman, T. W., Israel, M., & Arsura, M. (2004). ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Molecular and cellular biology, 24, 1823–1835.
Beg, A. A., & Baltimore, D. (1996). An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science (New York, N.Y.), 274, 782–784.
Oikawa, M., Wu, M., Lim, S., Knight, W. E., Miller, C. L., Cai, Y., Lu, Y., Blaxall, B. C., Takeishi, Y., Abe, J., & Yan, C. (2013). Cyclic nucleotide phosphodiesterase 3A1 protects the heart against ischemia-reperfusion injury. Journal of molecular and cellular cardiology, 64, 11–19.
Liu, J., Chen, C., Liu, Y., Sun, X., Ding, X., Qiu, L., Han, P., & James Kang, Y. (2018). Feature Article: Trientine selectively delivers copper to the heart and suppresses pressure overload-induced cardiac hypertrophy in rats. Experimental biology and medicine (Maywood, N.J.), 243, 1141–1152.
Zhang, J., Yu, L., Xu, Y., Liu, Y., Li, Z., Xue, X., Wan, S., & Wang, H. (2018). Long noncoding RNA upregulated in hypothermia treated cardiomyocytes protects against myocardial infarction through improving mitochondrial function. International journal of cardiology, 266, 213–217.
Zhang, J., Sheng, J., Dong, L., Xu, Y., Yu, L., Liu, Y., Huang, X., Wan, S., Lan, H. Y., & Wang, H. (2019). Cardiomyocyte-specific loss of RNA polymerase II subunit 5-mediating protein causes myocardial dysfunction and heart failure. Cardiovascular research, 115, 1617–1628.
Teringova, E., & Tousek, P. (2017). Apoptosis in ischemic heart disease. Journal of translational medicine, 15, 87.
Xia, P., Liu, Y., & Cheng, Z. (2016). Signaling Pathways in Cardiac Myocyte Apoptosis. BioMed research international, 2016, 9583268.
Beug, S. T., Cheung, H. H., LaCasse, E. C., & Korneluk, R. G. (2012). Modulation of immune signalling by inhibitors of apoptosis. Trends in immunology, 33, 535–545.
Ahmad, F., Lal, H., Zhou, J., Vagnozzi, R. J., Yu, J. E., Shang, X., Woodgett, J. R., Gao, E., & Force, T. (2014). Cardiomyocyte-specific deletion of Gsk3α mitigates post-myocardial infarction remodeling, contractile dysfunction, and heart failure. Journal of the American College of Cardiology, 64, 696–706.
Chatterjee, S., Stewart, A. S., Bish, L. T., Jayasankar, V., Kim, E. M., Pirolli, T., Burdick, J., Woo, Y. J., Gardner, T. J., & Sweeney, H. L. (2002). Viral gene transfer of the antiapoptotic factor Bcl-2 protects against chronic postischemic heart failure. Circulation, 106, I212–I217.
Czabotar, P. E., Lessene, G., Strasser, A., & Adams, J. M. (2014). Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews. Molecular cell biology, 15, 49–63.
Hochhauser, E., Cheporko, Y., Yasovich, N., Pinchas, L., Offen, D., Barhum, Y., Pannet, H., Tobar, A., Vidne, B. A., & Birk, E. (2007). Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell biochemistry and biophysics, 47, 11–20.
Astrada, S., Gomez, Y., Barrera, E., Obal, G., Pritsch, O., Pantano, S., Vallespí, M. G., & Bollati-Fogolín, M. (2016). Comparative analysis reveals amino acids critical for anticancer activity of peptide CIGB-552. Journal of peptide science : an official publication of the European Peptide Society, 22, 711–722.
Acknowledgements
This work was supported in part by National Science Foundation of China (Grant Number 81230004 to YJ Kang). The authors thank Qin Sheng, Xin Song, and Qipu Feng for technical support.
Author information
Authors and Affiliations
Contributions
All authors participated in the experimental design, interpretation of the results, and review of the manuscript; CL and HXP involved in the experimentation; CL performed the data analysis; YJK and CL wrote the manuscript; and YJK edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Author Y James Kang is a member of the Editorial Board for Cardiovascular Toxicology. The paper was handled by the other Editor and has undergone rigorous peer review process. Author Y James Kang was not involved in the journal’s review of, or decisions related to, this manuscript.
Additional information
Handling Editor: Kurt J. Varner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, C., Peng, H. & Kang, Y.J. Cardiomyocyte-Specific COMMD1 Deletion Suppresses Ischemia-Induced Myocardial Apoptosis. Cardiovasc Toxicol 21, 572–581 (2021). https://doi.org/10.1007/s12012-021-09650-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09650-5