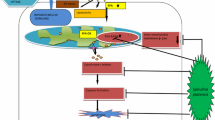
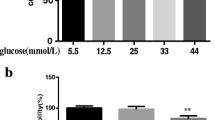
Abstract
Diabetic patients are known to have an independent risk of cardiomyopathy. Hyperglycemia leads to upregulation of reactive oxygen species (ROS) that may contribute to diabetic cardiomyopathy. Thus, agents that suppress glucose-induced intracellular ROS levels can have therapeutic potential against diabetic cardiomyopathy. Syzygium cumini is well known for its anti-diabetic potential, but its cardioprotective properties have not been evaluated yet. The aim of the present study is to analyze cardioprotective properties of methanolic seed extract (MSE) of S. cumini in diabetic in vitro conditions. ROS scavenging activity of MSE was studied in glucose-stressed H9C2 cardiac myoblasts after optimizing the safe dose of glucose and MSE by 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide. 2′,7′-dichlorfluorescein diacetate staining and Fluorescence-activated cell sorting analysis confirmed the suppression of ROS production by MSE in glucose-induced cells. The intracellular NO and H2O2 radical–scavenging activity of MSE was found to be significantly high in glucose-induced cells. Exposure of glucose-stressed H9C2 cells to MSE showed decline in the activity of catalase and superoxide dismutase enzymes and collagen content. 4′,6-diamidino-2-phenylindole, propidium iodide and 10-N-nonyl-3,6-bis (dimethylamino) acridine staining revealed that MSE protects myocardial cells from glucose-induced stress. Taken together, our findings revealed that the well-known anti-diabetic S. cumini can also protect the cardiac cells from glucose-induced stress.








Similar content being viewed by others
References
Banerjee, A., Dasgupta, N., & De, B. (2005). In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chemistry, 90, 727–733.
Valko, M., Leibfritz, D., Moncol, J., Mark, T. D., Cronin, M. T. D., Mazur, M., et al. (2007). Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry & Cell Biology, 39, 44–84.
Neill, S., Desikan, R., & Hancock, J. (2002). Hydrogen peroxide signaling. Current Opinion in Plant Biology, 5, 388–395.
Kangralkar, V. A., Patil, S. D., & Bandivadekar, R. M. (2010). Oxidative stress and diabetes: A review. International Journal of Pharmaceutical Applications, 1, 38–45.
Laakso, M. (1999). Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes, 48, 937–942.
Panicker, G. K., Karnad, D. R., Salvi, V., & Kothari, S. (2012). Cardiovascular risk of oral antidiabetic drugs: Current evidence and regulatory requirements for new drugs. Journal of the Association of Physicians of India, 60, 56–61.
Devasagayam, T. P., Tilak, J. C., Boloor, K. K., Sane, K. S., Ghaskadbi, S. S., & Lele, R. D. (2004). Free radicals and antioxidants in human health: Current status and future prospects. Journal of the Association of Physicians of India, 52, 794–804.
Vardi, M., Blum, S., & Levy, A. P. (2012). Haptoglobin genotype and cardiovascular outcomes in diabetes mellitus natural history of the disease and the effect of vitamin E treatment. Meta-analysis of the medical literature. European Journal of Internal Medicine, 23, 628–632.
Myung, S. K., Ju, W., Cho, B., Oh, S. W., Park, S. M., Koo, B. K., et al. (2013). Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. British Medical Journal, 18(346), f10.
Saikat, S., & Raja, C. (2011). Oxidative stress: Diagnostics, prevention, and therapy in the role of antioxidants in human health (ACS symposium series) (pp. 1–37). Washington DC: American Chemical Society.
Hill, M. F. (2008). Emerging role for antioxidant therapy in protection against diabetic cardiac complications: Experimental and clinical evidence for utilization of classic and new antioxidants. Current Cardiology Reviews, 4, 259–268.
Pandey, K. B., & Rizvi, S. I. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity, 2, 270–278.
Bopp, A., DeBona, K. S., Belle, L. P., Moresco, R. N., & Moretto, M. B. (2009). Syzygium cumini inhibits adenosine deaminase activity and reduces glucose levels in hyperglycemic patients. Fundamental & Clinical Pharmacology, 23, 501–507.
Ponnusamy, S., Ravindran, R., Zinjarde, S., Bhargava, S., & Ravi Kumar, A. (2011). Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evidence Based Complementary and Alternative Medicine,. doi:10.1155/2011/515647.
Saravanan, G., & Pari, L. (2008). Hypoglycemic and antihyperglycemic effect of Syzygium cumini bark in strptozotocin induced diabetic rats. Journal of Pharmacology and Toxicology, 3, 1–10.
Helmstädter, A. (2008). Syzygium cumini (L.) SKEELS (Myrtaceae) against diabetes–125 years of research. Pharmazie, 63, 91–101.
Baliga, M. S., Bhat, H. P., Baliga, B. R. V., Wilson, R., & Palatty, P. L. (2011). Phytochemistry, traditional uses and pharmacology of Eugenia jambolana Lam. (black plum): A review. Food Research International, 44, 1776–1789.
Ayyanar, M., & Subash-Babu, P. (2012). Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine, 2, 240–246.
Shoba, B. (2012). Antibacterial, phytochemical analysis of water extract of Syzygium cumini and analytical study by HPLC. Asian Journal of Experimental Biological Sciences, 3, 320–324.
Bhowmik, D., Gopinath, H., Pragati Kumar, B., Duraivel, S., Aravind, G., & Sampath Kumar, K. P. (2012). Traditional and medicinal uses of Indian black berry. Journal of Pharmacognosy Phytochemistry, 1, 37–42.
Moresco, R. N., Sperotto, R. L., Bernardi, A. S., Cardoso, R. F., & Gomes, P. (2007). Effect of the aqueous extract of Syzygium cumini on carbon tetrachloride-induced hepatotoxicity in rats. Phytotherapy Research, 21, 793–795.
Atale, N., Jaiswal, A., Chhabra, A., Malhotra, U., Kohli, S., Mohanty, S., et al. (2011). Phytochemical and antioxidant screening of Syzygium cumini seed extracts: A comparative study. Journal of Pharmaceutical Research, 4, 4530–4532.
Mastan, S. K., Chaitanya, G., Bhavya Latha, T., Srikanth, A., Sumalatha, G., & Eswar Kumar, K. (2009). Cardioprotective effect of methanolic extract of Syzygium cumini seeds on isoproterenol-induced myocardial infarction in rats. Der Pharmacia Lettre, 1, 143–149.
Sreejit, P., Kumar, S., & Verma, R. S. (2008). An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cellular and Developmental Biology Animal, 44, 45–50.
Brownlee, M. (2001). Biochemistry and molecular cell biology of diabetic complications. Nature, 414, 813–820.
Green, K., Brand, M. D., & Murphy, M. P. (2004). Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes, 53, S110–S118.
Ferrari, M., Fornasiero, M. C., & Isetta, A. M. (1990). MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. Journal of Immunological Methods, 131, 165–172.
Hescheler, J., Meyer, R., Plant, S., Krautwurst, D., Rosenthal, W., & Schultz, G. (1991). Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9C2) line from rat heart. Circulation Research, 69, 1476–1486.
Barcia, J. J. (2007). The giemsa stain: Its history and applications. International Journal of Surgical Pathology, 15, 292–296.
Wang, H., & Joseph, J. A. (1999). Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biology and Medicine, 27, 612–616.
Marotta, M., & Martino, G. (1985). Sensitive spectrophotomeric method for the quantitative estimation of collagen. Analytical Biochemistry, 150, 86–90.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Marcocci, L., Magguire, J. J., Droy-Lefaix, M. T., & Packer, L. (1994). The nitric oxide-scavenger properties of Ginkgo biloba extract EGB 761. Biochemical and Biophysical Research Communications, 15, 462–475.
Ruch, R. J., Cheng, S. J., & Klaunig, J. E. (1989). Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 10, 1003–1008.
Aebi, H. E. (1983). Catalase. In H. U. Bergmeyer (Ed.), Methods of enzymatic analysis (pp. 273–286). Weinheim: Verlag Chemie.
Beauchamp, C., & Fridovich, I. (1971). Super oxide dismutase: Improved assays and assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
Kapuscinski, J., & Skoczylas, B. (1978). Fluorescent complexes of DNA with DAPI (4′,6-diamidine-2-phenyl indole dihydrochloride) or DCI (4′,6- dicarboxyamide-2-phenyl indole). Nucleic Acids Research, 5, 3775–3799.
Brana, C., Benham, C., & Sundstrom, L. (2002). A method for characterising cell death in vitro by combining propidium iodide staining with immunohistochemistry. Brain Research Protocols, 10, 109–114.
Petit, J. M., Maftah, A., Ratinaud, M. H., & Julien, R. (1992). 10 N-Nonyl acridine orange interacts with cardiolipin and allows the quantification of this phospholipid in isolated mitochondria. European Journal of Biochemistry, 209(267–273), 40.
Perelman, A., Wachtel, C., Cohen, M., Haupt, S., Shapiro, H., & Tzur, A. (2012). JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death and Disease, 3. doi:10.1038/cddis.2012.171.
Tao, L. S., Mackenzie, C. R., & Charlson, M. E. (2008). Predictors of postoperative complications in the patient with diabetes mellitus. Journal of Diabetes and Its Complications, 22, 24–28.
Lamblin, N., Fertin, M., De-Groote, P., & Bauters, C. (2012). Cardiac remodeling and heart failure after a first anterior myocardial infarction in patients with diabetes mellitus. Journal of Cardiovascular Medicine, 13, 353–359.
Ku, P. M., Chen, L. J., Liang, J. R., Cheng, K. C., Li, Y. A., & Cheng, J. T. (2011). Molecular role of GATA binding protein 4 (GATA-4) in hyperglycemia-induced reduction of cardiac contractility. Cardiovascular Diabetology, 10, 1–15.
Ansley, D. M., & Wang, B. (2013). Oxidative stress and myocardial injury in the diabetic heart. Journal of Pathology, 229, 232–241.
Ahuja, P., Sdek, P., & MacLellan, W. R. (2007). Cardiacmyocyte cell cycle control in development, disease and regeneration. Physiological Reviews, 87, 521–544.
Feng, B., Chen, S., Chiu, J., George, B., & Chakrabarti, S. (2008). Regulation of cardiomyocyte hypertrophy in diabetes at the transcriptional level. American Journal of Physiology Endocrinology and Metabolism, 294, E1119–E1126.
Watkins, S. J., Borthwick, G. M., & Arthur, H. M. (2011). The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In vitro Cellular Developmental Biology Animal, 47, 125–131.
Seddon, M., Looi, Y. H., & Shah, A. M. (2007). Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart, 93, 903–907.
Umadevi, S., Gopi, V., Simna, S. P., Parthasarathy, A., Yousuf, S. M. J., & Elangovan, V. (2012). Studies on the cardio protective role of gallic acid against AGE-induced cell proliferation and oxidative stress in H9C2 (2–1) cells. Cardiovascular Toxicology, 12, 304–311.
Tsutsui, H., Kinugawa, S., & Matsushima, S. (2009). Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovascular Research, 81, 449–456.
Glucose in cell structure. Available from http://www.sigmaaldrich.com/ life-science/cell-culture/learning-center/media expert/glucose.html.
Farhangkhoee, H., Khan, Z. A., Chen, S., & Chakrabarti, S. (2006). Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutrition and Metabolism, 3, 1–18.
Zhang, G. X., Kimura, S., Murao, K., Obata, K., Matsuyoshi, H., & Takaki, M. (2010). Inhibition of cytochrome c release by 10-N-nonyl acridine orange, a cardiolipin-specific dye, during myocardial ischemia-reperfusion in the rat. American Journal of Physiology Heart and Circulatory Physiology, 298, H433–H439.
Caglayan, E., Stauber, B., Collins, A. R., Lyon, C. J., Yin, F., Liu, J., et al. (2008). Differential roles of cardiomyocyte and macrophage peroxisome proliferators-activated receptor gamma in cardiac fibrosis. Diabetes, 57, 2470–2479.
Acknowledgments
We acknowledge Jaypee Institute of Information Technology, Deemed to be University for providing the infrastructural support.
Conflict of interest
Authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atale, N., Chakraborty, M., Mohanty, S. et al. Cardioprotective Role of Syzygium cumini Against Glucose-Induced Oxidative Stress in H9C2 Cardiac Myocytes. Cardiovasc Toxicol 13, 278–289 (2013). https://doi.org/10.1007/s12012-013-9207-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-013-9207-1