Abstract
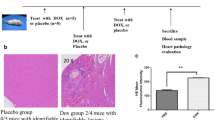
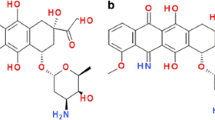
The use of anthracycline anticancer drugs is limited by a cumulative, dose-dependent cardiac toxicity. Iron chelation has long been considered as a promising strategy to limit this unfavorable side effect, either by restoring the disturbed cellular iron homeostasis or by removing redox-active iron, which may promote anthracycline-induced oxidative stress. Aroylhydrazone lipophilic iron chelators have shown promising results in the rabbit model of daunorubicin-induced cardiomyopathy as well as in cellular models. The lack of interference with the antiproliferative effects of the anthracyclines also favors their use in clinical settings. The dose, however, should be carefully titrated to prevent iron depletion, which apparently also applies for other strong iron chelators. We have shown that a mere ability of a compound to chelate iron is not the sole determinant of a good cardioprotector and the protective potential does not directly correlate with the ability of the chelators to prevent hydroxyl radical formation. These findings, however, do not weaken the role of iron in doxorubicin cardiotoxicity as such, they rather appeal for further investigations into the molecular mechanisms how anthracyclines interact with iron and how iron chelation may interfere with these processes.



Similar content being viewed by others
References
Mladenka, P., Simunek, T., Hubl, M., & Hrdina, R. (2006). The role of reactive oxygen and nitrogen species in cellular iron metabolism. Free Radical Research, 40(3), 263–272.
Walter, P. B., Knutson, M. D., Paler-Martinez, A., Lee, S., Xu, Y., Viteri, F. E., & Ames, B. N. (2002). Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proceedings of the National Academy of Sciences of the United States of America, 99(4), 2264–2269.
Minotti, G., Ronchi, R., Salvatorelli, E., Menna, P., & Cairo, G. (2001). Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Research, 61, 8422–8428.
Hrdina, R., Gersl, V., Klimtova, I., Simunek, T., Machackova, J., & Adamcova, M. (2000). Anthracycline-induced cardiotoxicity. Acta Medica (Hradec Kralove), 43(3), 75–82.
Kwok, J. C., & Richardson, D. R. (2003). Anthracyclines induce accumulation of iron in ferritin in myocardial and neoplastic cells: Inhibition of the ferritin iron mobilization pathway. Molecular Pharmacology, 63, 849–861.
Simunek, T., Sterba, M., Holeckova, M., Kaplanova, J., Klimtova, I., Adamcova, M., Gersl, V., & Hrdina, R. (2005). Myocardial content of selected elements in experimental anthracycline-induced cardiomyopathy in rabbits. Biometals, 18(2), 163–169.
Hasinoff, B. B., Schnabl, K. L., Marusak, R. A., Patel, D., & Huebner, E. (2003). Dexrazoxane (ICRF-187) protects cardiac myocytes against doxorubicin by preventing damage to mitochondria. Cardiovascular Toxicology, 3(2), 89–99.
Voest, E. E., van Acker, S. A., van der Vijgh, W. J., van Asbeck, B. S., & Bast, A. (1994). Comparison of different iron chelators as protective agents against acute doxorubicin-induced cardiotoxicity. Journal of Molecular and Cellular Cardiology, 26(9), 1179–1185.
Barnabe, N., Zastre, J. A., Venkataram, S., & Hasinoff, B. B. (2002). Deferiprone protects against doxorubicin-induced myocyte cytotoxicity. Free Radical and Biology Medicine, 33(2), 266–275.
Link, G., Tirosh, R., Pinson, A., & Hershko, C. (1996). Role of iron in the potentiation of anthracycline cardiotoxicity: Identification of heart cell mitochondria as a major site of iron-anthracycline interaction. The Journal of Laboratory and Clinical Medicine, 127(3), 272–278.
Hasinoff, B. B., Patel, D., & Wu, X. (2003). The oral iron chelator ICL670A (deferasirox) does not protect myocytes against doxorubicin. Free Radical and Biology Medicine, 35(11), 1469–1479.
Simunek, T., Klimtova, I., Kaplanova, J., Mazurova, Y., Adamcova, M., Sterba, M., Hrdina, R., & Gersl, V. (2004). Rabbit model for in vivo study of anthracycline-induced heart failure and for the evaluation of protective agents. European Journal of Heart Failure, 6(4), 377–387.
Richardson, D. R., Tran, E. H., & Ponka, P. (1995). The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. Blood, 86, 4295–4306.
Kovarikova, P., Klimes, J., Sterba, M., Popelova, O., Gersl, V., & Ponka, P. (2006). HPLC determination of novel aroylhydrazone iron chelator (o-108) in rabbit plasma and its application to a pilot pharmacokinetic study. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 838, 107–112.
Kovarikova, P., Klimes, J., Sterba, M., Popelova, O., Mokry, M., Gersl, V., & Ponka, P. (2005). Development of high-performance liquid chromatographic determination of salicylaldehyde isonicotinoyl hydrazone in rabbit plasma and application of this method to an in vivo study. Journal of Seperation Science, 28, 1300–1306.
Charkoudian, L. K., Pham, D. M., & Franz, K. J. (2006). A pro-chelator triggered by hydrogen peroxide inhibits iron-promoted hydroxyl radical formation. Journal of the American Chemical Society, 128(38), 12424–12425.
Simunek, T., Boer, C., Bouwman, R. A., Vlasblom, R., Versteilen, A. M., Sterba, M., Gersl, V., Hrdina, R., Ponka, P., de Lange, J. J., Paulus, W. J., & Musters, R. J. (2005). SIH–––a novel lipophilic iron chelator–protects H9c2 cardiomyoblasts from oxidative stress-induced mitochondrial injury and cell death. Journal of Molecular and Cellular Cardiology, 39(2), 345–354.
Schroterova, L., Kaiserova, H., Baliharova, V., Velik, J., Gersl, V., & Kvasnickova, E. (2004). The effect of new lipophilic chelators on the activities of cytosolic reductases and P450 cytochromes involved in the metabolism of anthracycline antibiotics: studies in vitro. Physiological Research, 53(6), 683–691.
Kaiserova, H., den Hartog, G. J., Simunek, T., Schroterova, L., Kvasnickova, E., & Bast, A. (2006). Iron is not involved in oxidative stress-mediated cytotoxicity of doxorubicin and bleomycin. British Journal of Pharmacology, 149(7), 920–930.
Sterba, M., Popelova, O., Simunek, T., Mazurova, Y., Potacova, A., Adamcova, M., Kaiserova, H., Ponka, P., & Gersl, V. (2006). Cardioprotective effects of a novel iron chelator, pyridoxal 2-chlorobenzoyl hydrazone, in the rabbit model of daunorubicin-induced cardiotoxicity. The Journal of Pharmacology and Experimental Therapeutics, 319(3), 1336–1337.
Simunek, T., Klimtova, I., Kaplanova, J., Sterba, M., Mazurova, Y., Adamcova, M., Hrdina, R., Gersl, V., & Ponka, P. (2005). Study of daunorubicin cardiotoxicity prevention with pyridoxal isonicotinoyl hydrazone in rabbits. Pharmacology Research, 51(3), 223–231.
Bast, A., Kaiserova, H., den Hartog, G. J., Haenen, G. R., van der Vijgh1, W. J. (2007). Protectors against doxorubicin-induced cardiotoxicity: Flavonoids. Cell Biology and Toxicology, 23(1), 39–47.
Hershko, C., Link, G., Tzahor, M., Kaltwasser, J. P., Athias, P., Grynberg, A., & Pinson, A. (1993). Anthracycline toxicity is potentiated by iron and inhibited by deferoxamine: Studies in rat heart cells in culture. The Journal of Laboratory and Clinical Medicine, 122(3), 245–251.
Herman, E. H., Zhang, J., & Ferrans, V. J. (1994). Comparison of the protective effects of desferrioxamine and ICRF-187 against doxorubicin-induced toxicity in spontaneously hypertensive rats. Cancer Chemotherapy and Pharmacology, 35(2), 93–100.
Saad, S. Y., Najjar, T. A., & Al-Rikabi, A. C. (2001). The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacology Research, 43(3), 211–218.
Acknowledgments
The authors were supported by grants GAUK 97/2005, GAČR 305/05/P156, and a Research Project MSM0021620820.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaiserová, H., Šimůnek, T., Štěrba, M. et al. New iron chelators in anthracycline-induced cardiotoxicity. Cardiovasc Toxicol 7, 145–150 (2007). https://doi.org/10.1007/s12012-007-0020-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-007-0020-6