Abstract
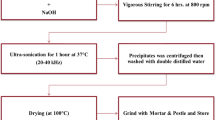
Titanium dioxide (TiO2) nanoparticles are promising biomedical agents characterized by good biocompatibility. In this study, we explored the cytotoxicity of TiO2−x nanoparticles with a different Ti3+(Ti2+)/Ti4+ ratio and analyzed the efficiency of eryptosis indices as a tool in nanotoxicology. Two types of TiO2−x nanoparticles (NPs) were synthesized by the hydrolysis of titanium alkoxide varying the nitric acid content in the hydrolysis mixture. Transmission electron microscopy (TEM) images show that 1-TiO2−x and 2-TiO2−x NPs are 5 nm in size, whereas X-ray photoelectron spectroscopy (XPS) reveals different Ti3+ (Ti2+)/Ti4+ ratios in the crystal lattices of synthesized NPs. 1-TiO2−x nanoparticles contained 54% Ti4+, 38% Ti3+, and 8% Ti2+, while the relative amount of Ti4+ and Ti3+ in the crystal lattice of 2-TiO2−x nanoparticles was 63% and 37%, respectively. Cell viability and cell motility induced by TiO2−x nanoparticles were investigated on primary fibroblast cultures. Eryptosis modulation by the nanoparticles along with cell death mechanisms was studied on rat erythrocytes. We report that both TiO2−x nanoparticles do not decrease the viability of fibroblasts simultaneously stimulating cell migration. Data from in vitro studies on erythrocytes indicate that TiO2−x nanoparticles trigger eryptosis via ROS- (1-TiO2−x) and Ca2+-mediated mechanisms (both TiO2−x nanoparticles) suggesting that evaluation of eryptosis parameters is a more sensitive nanotoxicological approach for TiO2−x nanoparticles than cultured fibroblast assays. TiO2−x nanoparticles are characterized by low toxicity against fibroblasts, but they induce eryptosis, which is shown to be a promising tool for nanotoxicity screening. The Ti3+ (Ti2+)/Ti4+ ratio at least partly determines the cytotoxicity mechanisms for TiO2−x nanoparticles.









Similar content being viewed by others
Data Availability
Raw data were generated at Kharkiv National Medical University and Institute for Scintillation Materials of the National Academy of Sciences of Ukraine. Derived data that support the findings of this research are available from the corresponding author Anton Tkachenko on request.
References
Abdel-Mageed HM, AbuelEzz NZ, Radwan RA, Mohamed SA (2021) Nanoparticles in nanomedicine: a comprehensive updated review on current status, challenges and emerging opportunities. J Microencapsul 38(6):414–436. https://doi.org/10.1080/02652048.2021.1942275
Jafari S, Mahyad B, Hashemzadeh H, Janfaza S, Gholikhani T, Tayebi L (2020) Biomedical applications of TiO2 nanostructures: recent advances. Int J Nanomedicine 15:3447–3470. https://doi.org/10.2147/IJN.S249441
Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT, Glowacka-Sobotta A, Stanisz B, Goslinski T, Sobotta L (2020) Titanium dioxide nanoparticles: prospects and applications in medicine. Nanomaterials (Basel) 10(2):387. https://doi.org/10.3390/nano10020387
Çeşmeli S, Biray Avci C (2019) Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J Drug Target 27(7):762–766. https://doi.org/10.1080/1061186X.2018.1527338
Behnam MA, Emami F, Sobhani Z, Dehghanian AR (2018) The application of titanium dioxide (TiO2) nanoparticles in the photo-thermal therapy of melanoma cancer model. Iran J Basic Med Sci 21(11):1133–1139. https://doi.org/10.22038/IJBMS.2018.30284.7304
Abou-Yousef H, Dacrory S, Hasanin M, Saber E, Kamel S (2021) Biocompatible hydrogel based on aldehyde-functionalized cellulose and chitosan for potential control drug release. Sustain Chem Pharm 21:100419. https://doi.org/10.1016/j.scp.2021.100419
Ali OM, Hasanin MS, Suleiman WB, El-Husseiny Helal E, Hashem AH (2022) Green biosynthesis of titanium dioxide quantum dots using watermelon peel waste: antimicrobial, antioxidant, and anticancer activities. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-022-02772-y.
El-Naggar ME, Hasanin M, Youssef AM, Aldalbahi A, El-Newehy MH, Abdelhameed RM (2020) Hydroxyethyl cellulose/bacterial cellulose cryogel dopped silver@titanium oxide nanoparticles: antimicrobial activity and controlled release of Tebuconazole fungicide. Int J Biol Macromol 165(Pt A):1010–1021. https://doi.org/10.1016/j.ijbiomac.2020.09.226
Hasanin MS, Abdelhameed RM, Dacrory S, Abou-Yousef H, Kamel S (2021) Photocatalytic degradation of pesticide intermediate using green eco-friendly amino functionalized cellulose nanocomposites. Mater Sci Eng B 270:115231. https://doi.org/10.1016/j.mseb.2021.115231
Pan X, Yang M-Q, Fu X, Zhang N, Xu Y-J (2013) Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale 5:3601–3614. https://doi.org/10.1039/c3nr00476g
Sahlin H, Contreras R, Gaskill D, Bjursten L, Frangos J (2006) Anti-inflammatory properties of micropatterned titanium coatings. J Biomed Mater A 77:43–49
Suzuki R, Muyco J, McKittrick J, Frangos J (2003) Reactive oxygen species inhibited by titanium oxide coatings. J Biomed Mater A 66:396–402
Shakeel M, Jabeen F, Shabbir S, Asghar MS, Khan MS, Chaudhry AS (2016) Toxicity of nano-titanium dioxide (TiO2-NP) through various routes of exposure: a review. Biol Trace Elem Res 172(1):1–36. https://doi.org/10.1007/s12011-015-0550-x
Chellappa M, Anjaneyulu U, Manivasagam G (2015) Vijayalakshmi U (2015) Preparation and evaluation of the cytotoxic nature of TiO2 nanoparticles by direct contact method. Int J Nanomedicine 10(1):31–41. https://doi.org/10.2147/IJN.S79978
Grande F, Tucci P (2016) Titanium dioxide nanoparticles: a risk for human health? Mini Rev Med Chem 16(9):762–769. https://doi.org/10.2174/1389557516666160321114341
Li M, Yin JJ, Wamer WG, Lo YM (2014) Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J Food Drug Anal 22(1):76–85. https://doi.org/10.1016/j.jfda.2014.01.006
Kong L, Barber T, Aldinger J, Bowman L, Leonard S, Zhao J, Ding M (2022) ROS generation is involved in titanium dioxide nanoparticle-induced AP-1 activation through p38 MAPK and ERK pathways in JB6 cells. Environ Toxicol 37(2):237–244. https://doi.org/10.1002/tox.23393
Hu H, Fan X, Yin Y, Guo Q, Yang D, Wei X, Zhang B, Liu J, Wu Q, Oh Y, Chen K, Feng Y, Hou L, Li L, Gu N (2019) Mechanisms of titanium dioxide nanoparticle-induced oxidative stress and modulation of plasma glucose in mice. Environ Toxicol 34(11):1221–1235. https://doi.org/10.1002/tox.22823
Fu L, Hamzeh M, Dodard S, Zhao YH, Sunahara GI (2015) Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation. Environ Toxicol Pharmacol 39(3):1074–1080. https://doi.org/10.1016/j.etap.2015.03.015
Saquib Q, Al-Khedhairy AA, Siddiqui MA, Abou-Tarboush FM, Azam A, Musarrat J (2012) Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol In Vitro 26(2):351–361. https://doi.org/10.1016/j.tiv.2011.12.011
Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, Dhawan A (2011) ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro 25(1):231–241. https://doi.org/10.1016/j.tiv.2010.11.008
Xue C, Wu J, Lan F, Liu W, Yang X, Zeng F, Xu H (2010) Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J Nanosci Nanotechnol 10(12):8500–8507. https://doi.org/10.1166/jnn.2010.2682
Naserzadeh P, Ghanbary F, Ashtari P, Seydi E, Ashtari K, Akbari M (2018) Biocompatibility assessment of titanium dioxide nanoparticles in mice fetoplacental unit. J Biomed Mater Res A 106(2):580–589. https://doi.org/10.1002/jbm.a.36221
Gali NK, Ning Z, Daoud W, Brimblecombe P (2016) Investigation on the mechanism of non-photocatalytically TiO2-induced reactive oxygen species and its significance on cell cycle and morphology. J Appl Toxicol 36(10):1355–1363. https://doi.org/10.1002/jat.3341
Skocaj M, Filipic M, Petkovic J, Novak S (2011) Titanium dioxide in our everyday life; is it safe? Radiol Oncol 45(4):227–247. https://doi.org/10.2478/v10019-011-0037-0
Franchi LP, Manshian BB, de Souza TA, Soenen SJ, Matsubara EY, Rosolen JM, Takahashi CS (2015) Cyto- and genotoxic effects of metallic nanoparticles in untransformed human fibroblast. Toxicol In Vitro 29(7):1319–1331. https://doi.org/10.1016/j.tiv.2015.05.010
Sabbioni E, Fortaner S, Farina M, Del Torchio R, Olivato I, Petrarca C, Bernardini G, Mariani-Costantini R, Perconti S, Di Giampaolo L, Gornati R, Di Gioacchino M (2014) Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: an in vitro model. Nanotoxicology 8(4):455–464. https://doi.org/10.3109/17435390.2013.796538
Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG, Schiffmann D (2002) Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Perspect 110(8):797–800. https://doi.org/10.1289/ehp.02110797
Forest V (2022) Experimental and computational nanotoxicology-complementary approaches for nanomaterial hazard assessment. Nanomaterials (Basel) 12(8):1346. https://doi.org/10.3390/nano12081346
Tirumala MG, Anchi P, Raja S, Rachamalla M, Godugu C (2021) Novel methods and approaches for safety evaluation of nanoparticle formulations: a focus towards in vitro models and adverse outcome pathways. Front Pharmacol 12:612659. https://doi.org/10.3389/fphar.2021.612659
Onishchenko A, Myasoedov V, Yefimova S, Nakonechna O, Prokopyuk V, Butov D, Kökbaş U, Klochkov V, Maksimchuk P, Kavok N, Tkachenko A (2022) UV light-activated GdYVO4:Eu3+ nanoparticles induce reactive oxygen species generation in leukocytes without affecting erythrocytes in vitro. Biol Trace Elem Res 200(6):2777–2792. https://doi.org/10.1007/s12011-021-02867-z
Barzegar S, Rezvani MR, Safa M, Amani A, Abbaspour A, Pourfathollah A, Hashemi J, Zaker F (2021) Dose-dependent efficacy of antioxidant nanoparticles on red blood cells storage. J Educ Health Promot 10:256. https://doi.org/10.4103/jehp.jehp_1638_20
Bigdelou P, Vahedi A, Kiosidou E, Farnoud AM (2020) Loss of membrane asymmetry alters the interactions of erythrocytes with engineered silica nanoparticles. Biointerphases 15(4):041001. https://doi.org/10.1116/6.0000246
Ferdous Z, Beegam S, Tariq S, Ali BH, Nemmar A (2018) The in vitro effect of polyvinylpyrrolidone and citrate coated silver nanoparticles on erythrocytic oxidative damage and eryptosis. Cell Physiol Biochem 49(4):1577–1588. https://doi.org/10.1159/000493460
Xu D, Ran Q, Xiang Y, Linhai J, Smith BM, Bou-Abdallah F, Lund R, Li Z, Dong H (2016) Toward hemocompatible self-assembling antimicrobial nanofibers: understanding the synergistic effect of supramolecular structure and PEGylation on hemocompatibility. RSC Adv 6(19):15911–15919. https://doi.org/10.1039/C5RA24553B
Ran Q, Xiang Y, Liu Y, Xiang L, Li F, Deng X, Xiao Y, Chen L, Chen L, Li Z (2015) Eryptosis indices as a novel predictive parameter for biocompatibility of Fe3O4 magnetic nanoparticles on erythrocytes. Sci Rep 5:16209. https://doi.org/10.1038/srep16209
Lau IP, Chen H, Wang J, Ong HC, Leung KC, Ho HP, Kong SK (2012) In vitro effect of CTAB- and PEG-coated gold nanorods on the induction of eryptosis/erythroptosis in human erythrocytes. Nanotoxicology 6:847–856. https://doi.org/10.3109/17435390.2011.625132
Wadhwa R, Aggarwal T, Thapliyal N, Kumar A, Priya Yadav P, Kumari V, Reddy BSC, Chandra P, Maurya PK (2019) Red blood cells as an efficient in vitro model for evaluating the efficacy of metallic nanoparticles. 3 Biotech 9(7):279
Farag MR, Alagawany M (2018) Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem Biol Interact 279:73–83. https://doi.org/10.1016/j.cbi.2017.11.007
O’Regan B, Moser J, Anderson M, Gratzel M (1990) Vectorial electron injection into transparent semiconductor membranes and electric field effects on the dynamics of light-induced charge separation. J Phys Chem 94:8720–8726. https://doi.org/10.1021/j100387a017
Alfhili MA, Alamri HS, Alsughayyir J, Basudan AM (2022) Induction of hemolysis and eryptosis by occupational pollutant nickel chloride is mediated through calcium influx and p38 MAP kinase signaling. Int J Occup Med Environ Health 35(1):1–11. https://doi.org/10.13075/ijomeh.1896.01814
Tkachenko A, Kot Y, Prokopyuk V, Onishchenko A, Bondareva A, Kapustnik V, Chumachenko T, Perskiy Y, Butov D, Nakonechna O (2022) Food additive E407a stimulates eryptosis in a dose-dependent manner. Wien Med Wochenschr 172:135–143. https://doi.org/10.1007/s10354-021-00874-2
Tkachenko A, Prokopiuk V, Onishchenko A (2022) Semi-refined carrageenan induces eryptosis in a Ca2+-dependent manner. J Clin Med Kaz 19(1):42–5. https://doi.org/10.23950/jcmk/11576
Prokopyuk M, Onishchenko A, Yefimova S, Chumachenko T, Kavok N, Maksimchuk P, Klochkov V, Tkachenko A (2021) Cytotoxicity tests on cultured rat skin fibroblasts revealed no toxicity for low concentrations of GdYVO4:Eu3+ nanoparticles. IEEE 11th International Conference Nanomaterials: Applications & Properties (NAP):1–4. https://doi.org/10.1109/NAP51885.2021.9568547
Kumar P, Nagarajan A, Uchil PD (2018) Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc 2018(6). https://doi.org/10.1101/pdb.prot095505.
Chang Y, Guo K, Li Q, Li C, Guo Z, Li H (2016) Multiple directional differentiation difference of neonatal rat fibroblasts from six organs. Cell Physiol Biochem 39(1):157–171. https://doi.org/10.1159/000445613
Repetto G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3(7):1125–1131. https://doi.org/10.1038/nprot.2008.75
Hulkower KI, Herber RL (2011) Cell migration and invasion assays as tools for drug discovery. Pharmaceutics 3(1):107–124. https://doi.org/10.3390/pharmaceutics3010107
Rashid MM, Forte Tavčer P, Tomšič B (2021) Influence of titanium dioxide nanoparticles on human health and the environment. Nanomaterials (Basel) 11(9):2354. https://doi.org/10.3390/nano11092354
Shabbir S, Kulyar MF, Bhutta ZA, Boruah P, Asif M (2021) Toxicological consequences of titanium dioxide nanoparticles (TiO2 NPs) and their jeopardy to human population. Bionanoscience 11(2):621–632. https://doi.org/10.1007/s12668-021-00836-3
Baranowska-Wójcik E, Szwajgier D, Oleszczuk P, Winiarska-Mieczan A (2020) Effects of titanium dioxide nanoparticles exposure on human health—a review. Biol Trace Elem Res 193(1):118–129. https://doi.org/10.1007/s12011-019-01706-6
You C, Li Q, Wang X, Wu P, Ho JK, Jin R, Zhang L, Shao H, Han C (2017) Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation. Sci Rep 7(1):10489. https://doi.org/10.1038/s41598-017-10481-0
Ismail NA, Amin KAM, Majid FAA, Razali MH (2019) Gellan gum incorporating titanium dioxide nanoparticles biofilm as wound dressing: physicochemical, mechanical, antibacterial properties and wound healing studies. Mater Sci Eng C Mater Biol Appl 103:109770. https://doi.org/10.1016/j.msec.2019
Javanmardi S, Ghojoghi A, Divband B, Ashrafi J (2018) Titanium dioxide nanoparticle/gelatin: a potential burn wound healing biomaterial. Wounds 30(12):372–379
Nikpasand A, Parvizi MR (2019) Evaluation of the effect of titatnium dioxide nanoparticles/gelatin composite on infected skin wound healing; an animal model study. Bull Emerg Trauma 7(4):366–372. https://doi.org/10.29252/beat-070405
Yu Z, Li Q, Wang J, Yu Y, Wang Y, Zhou Q, Li P (2020) Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res Lett 15(1):115. https://doi.org/10.1186/s11671-020-03344-7
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int 2013:942916. https://doi.org/10.1155/2013/942916
Föller M, Lang F (2020) Ion transport in eryptosis, the suicidal death of erythrocytes. Front Cell Dev Biol 8:597. https://doi.org/10.3389/fcell.2020.00597
Acknowledgements
This study was performed as a fragment of a research entitled “Development of methods for evaluating biosafety and bioeffects of xenobiotics using in vitro and in vivo models.”
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to this paper. Conceptualization: Anton Tkachenko, Anatolii Onishchenko; data analysis and original draft writing: Anton Tkachenko, Svetlana Yefimova, Volodymyr Prokopiuk; experimental data acquisition: Volodymyr Prokopiuk, Pavel Maksimchuk, Irina Bespalova, Antolii Onishchenko; interpretation of data: Anton Tkachenko, Svetlana Yefimova, Valeriy Kapustnik, Valeriy Myasoedov, Anatolii Onishchenko, Pavel Maksimchuk, Irina Bespalova; statistical analysis: Dmytro Butov, Volodymyr Prokopiuk; proofreading: Dmytro Butov, Anton Tkachenko, Svetlana Yefimova; funding: Anton Tkachenko, Valeriy Myasoedov, Valeriy Kapustnik. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Ethics Committee of Kharkiv National Medical University (minutes #3 dated August 28, 2020).
Consent for Publication
All authors have approved the submission.
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prokopiuk, V., Yefimova, S., Onishchenko, A. et al. Assessing the Cytotoxicity of TiO2−x Nanoparticles with a Different Ti3+(Ti2+)/Ti4+ Ratio. Biol Trace Elem Res 201, 3117–3130 (2023). https://doi.org/10.1007/s12011-022-03403-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03403-3