Abstract
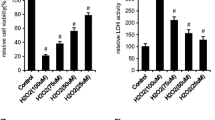
Aluminum (Al) inhibits osteoblast-mediated bone formation by oxidative stress, resulting in Al-induced bone disease. Melatonin (MT) has received extensive attention due to its antioxidant and maintenance of bone health effect. To evaluate the protective effect and mechanism of MT on AlCl3-induced osteoblast dysfunction, MC3T3-E1 cells were treated with MT (100 μM) and/or AlCl3 (8 μM). First, MT alleviated AlCl3-induced osteoblast dysfunction, presenting as the reduced apoptosis rate as well as increased cell viability, alkaline phosphatase (ALP) activity, and type I collagen (COL-1) level. Then, MT significantly attenuated AlCl3-induced oxidative stress, presenting as the reduced reactive oxygen species and 8-hydroxy-2′-deoxyguanosine levels as well as increased glutathione level and superoxide dismutase activity. Finally, MT protected MC3T3-E1 cells against p53-dependent apoptosis and differentiation suppression, as assessed by Caspase-3 activity, protein levels of p53, Bcl-2-associated X protein (Bax), B cell lymphoma gene 2 (Bcl-2), cytosolic Cytochrome c, Runt-related transcription factor 2 (Runx2), and Osterix, as well as the mRNA levels of Bax, Bcl-2, Runx2, Osterix, ALP, and COL-1. Overall, our findings demonstrate MT attenuates AlCl3-induced apoptosis and osteoblastic differentiation suppression by inhibiting oxidative stress in MC3T3-E1 cells.




Similar content being viewed by others
References
Klotz K, Weistenhöfer W, Neff F, Hartwig A, Van TC, Drexler H (2017) The health effects of aluminum exposure. Dtsch Arztebl Int 114:653–659
Cao C, Li X, Qin L, Luo J, Zhang M, Ou Z, Wang K (2018) High selenium yeast mitigates aluminum-induced cerebral inflammation by increasing oxidative stress and blocking NO production. Biometalst 31:835–843
Willhite CC, Karyakina NA, Yokel RA, Yenugadhati N, Wisniewski TM, Arnold IM, Momoli F, Krewski D (2014) Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol 44:1–80
Saiyed SM, Yokel RA (2005) Aluminium content of some foods and food products in the USA, with aluminium food additives. Food Addit Contam 22:234–244
Willhite CC, Ball GL, Mclellan CJ (2012) Total allowable concentrations of monomeric inorganic aluminum and hydrated aluminum silicates in drinking water. Crit Rev Toxicol 42:358–442
Priest ND (2004) The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. J Environ Monit 6:375–403
Koichi O, Kohei I, Yuki K, Yoshihiko M, Taku M, Yasumoto M (2010) Exposure assessment of metal intakes from drinking water relative to those from total diet in Japan. Water Sci Technol 62:2694–2701
Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Kacew S, Lindsay J, Mahfouz AM, Rondeau V (2007) Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev 10:11–269
Natalia VD, Raja S, Jennifer LV, Shaoyu Z, Jason U, Richard N (2012) The metal transporter SMF-3/DMT-1 mediates aluminum-induced dopamine neuron degeneration. J Neurochem 124:147–157
Malluche H (2002) Aluminium and bone disease in chronic renal failure. Nephrol Dial Transplant 17(Suppl 2):21–24
Assunção JH, Malavolta EA, Mec G, Filippi RZ, Neto FA (2017) Multifocal osteonecrosis secondary to occupational exposure to aluminum. Acta Ortop Bras 25:103–106
Andress DL, Maloney NA, Endres DB, Sherrard DJ (1986) Aluminum-associated bone disease in chronic renal failure: high prevalence in a long-term dialysis population. J Bone Miner Res 1:391–398
Marie PJ (2015) Osteoblast dysfunctions in bone diseases: from cellular and molecular mechanisms to therapeutic strategies. Cell Mol Life Sci 72:1347–1361
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297
Basu S, Michaelsson K, Olofsson H, Johansson S, Melhus H (2001) Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288:275–279
Yang X, Yu K, Wang H, Zhang H, Bai C, Song M, Han Y, Shao B, Li Y, Li X (2018) Bone impairment caused by AlCl3 is associated with activation of the JNK apoptotic pathway mediated by oxidative stress. Food Chem Toxicol 116:307–314
Kumar V, Gill KD (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41:154–166
Liang H, Gao J, Zhang C, Li C, Wang Q, Fan J, Wu Z, Wang Q (2019) Nicotinamide mononucleotide alleviates Aluminum induced bone loss by inhibiting the TXNIP-NLRP3 inflammasome. Toxicol Appl Pharmacol 362:20–27
Claustrat B, Leston L (2015) Melatonin: physiological effects in humans. Neurochirurgie 61:77–84
Li J, Zheng X, Ma X, Xu X, Du Y, Lv Q, Li X, Wu Y, Sun H, Yu L, Zhang Z (2019) Melatonin protects against chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2 pathway. J Biol Chem 197:110698
Fischer TW, Kleszczynski K, Hardkop LH, Kruse N, Zillikens D (2013) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res 54:303–312
Machida M, Dubousset J, Yamada T, Kimura J (2009) Serum melatonin levels in adolescent idiopathic scoliosis prediction and prevention for curve progression--a prospective study. J Pineal Res 46:344–348
Li T, Jiang S, Lu C, Yang W, Yang Z, Hu W, Xin Z, Yang Y (2019) Melatonin: another avenue for treating osteoporosis? J Pineal Res 66:e12548
Yildirimturk S, Batu S, Alatli C, Olgac V, Firat D, Sirin Y (2016) The effects of supplemental melatonin administration on the healing of bone defects in streptozotocin-induced diabetic rats. J Appl Oral Sci 24:239–249
Tresguerres IF, Tamimi F, Eimar H, Barralet JE, Prieto S, Torres J, Calvo-Guirado JL, Tresguerres JA (2014) Melatonin dietary supplement as an anti-aging therapy for age-related bone loss. Rejuvenation Res 17:341–346
Proksch S, Strobel SL, Vach K, Abouassi T, Tomakidi P, Ratka-Kruger P, Hellwig E (2014) Melatonin as a candidate therapeutic drug for protecting bone cells from chlorhexidine-induced damage. J Periodontol 85:379–389
Son JH, Cho YC, Sung IY, Kim IR, Park BS, Kim YD (2014) Melatonin promotes osteoblast differentiation and mineralization of MC3T3-E1 cells under hypoxic conditions through activation of PKD/p38 pathways. J Pineal Res 57:385–392
Yoo YM, Han TY, Kim HS (2016) Melatonin suppresses autophagy induced by clinostat in preosteoblast MC3T3-E1 cells. Int J Mol Sci 17:526
Yu H, Zhang J, Ji Q, Yu K, Wang P, Song M, Cao Z, Zhang X, Li Y (2019) Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol Environ Saf 173:131–141
Sadek KM, Lebda MA, Abouzed TK (2019) The possible neuroprotective effects of melatonin in aluminum chloride-induced neurotoxicity via antioxidant pathway and Nrf2 signaling apart from metal chelation. Environ Sci Pollut Res Int 26:9174–9183
Asghari MH, Moloudizargari M, Baeeri M, Baghaei A, Rahimifard M, Solgi R, Jafari A, Aminjan HH, Hassani S, Moghadamnia AA, Ostad SN, Abdollah M (2017) On the mechanisms of melatonin in protection of aluminum phosphide cardiotoxicity. Arch Toxicol 91:3109–3120
Yang X, Zhang J, Ji Q, Wang F, Song M, Li Y (2018) Autophagy protects MC3T3-E1 cells upon aluminum-induced apoptosis. Biol Trace Elem Res 185:1–7
Park KH, Kang JW, Lee EM, Kim JS, Rhee YH, Kim M, Jeong SJ, Park YG, Kim SH (2011) Melatonin promotes osteoblastic differentiation through the BMP/ERK/Wnt signaling pathways. J Pineal Res 51:187–194
Jiang X, Gu S, Liu D, Zhao L, Xia S, He X, Chen H, Ge J (2018) Lactobacillus brevis 23017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-kappaB signaling cascades. Front Microbiol 9:2425
Zhang B, Guo H, Yang W, Li M, Zou Y, Loor JJ, Xia C, Xu C (2019) Effects of ORAI calcium release-activated calcium modulator 1 (ORAI1) on neutrophil activity in dairy cows with subclinical hypocalcemia1. J Anim Sci 97:3326–3336
Wang J, Zhao Y, Cheng X, Li Y, Xu H, Manthari RK, Wang J (2018) Effects of different Ca2+ level on fluoride-induced apoptosis pathway of endoplasmic reticulum in the rabbit osteoblast in vitro. Food Chem Toxicol 116:189–195
Cao Z, Fu Y, Sun X, Zhang Q, Xu F, Li Y (2016) Aluminum trichloride inhibits osteoblastic differentiation through inactivation of Wnt/β-catenin signaling pathway in rat osteoblasts. Environ Toxicol Pharmacol 42:198–204
Xu F, Ren L, Song M, Shao B, Han Y, Cao Z, Li Y (2017) Fas- and mitochondria-mediated signaling pathway involved in osteoblast apoptosis induced by AlCl3. Biol Trace Elem Res 184:1–13
Sun X, Wang H, Huang W, Yu H, Shen T, Song M, Han Y, Li Y, Zhu Y (2017) Inhibition of bone formation in rats by aluminum exposure via Wnt/β-catenin pathway. Chemosphere 176:1–7
Kim HJ, Kim SH, Kim MS, Lee EJ, Oh HG, Oh WM, Park SW, Kim WJ, Lee GJ, Choi NG, Koh JT, Dinh DB, Hardin RR, Johnson K, Sylvia VL, Schmitz JP, Dean DD (2005) Varying Ti-6Al-4V surface roughness induces different early morphologic and molecular responses in MG63 osteoblast-like cells. J Biomed Mater Res A 74:366–373
Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG (2002) Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A 99:12600–12605
Xu L, Zhang L, Wang Z, Li C, Li S, Li L, Fan Q, Zheng L (2018) Melatonin suppresses estrogen deficiency-induced osteoporosis and promotes osteoblastogenesis by inactivating the NLRP3 inflammasome. Calcif Tissue Int 103:1–11
Zhu Y, Hu C, Zheng P, Miao L, Yan X, Li H, Wang Z, Gao B, Li Y (2016) Ginsenoside Rb1 alleviates aluminum chloride-induced rat osteoblasts dysfunction. Toxicology 368-369:183–188
Gurley KE, Moser R, Gu Y, Hasty P, Kemp CJ (2009) DNA-PK suppresses a p53-independent apoptotic response to DNA damage. EMBO Rep 10:87–93
Miyashita T, Harigai M, Hanada M, Reed JC (1994) Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res 54:3131–3135
Wang X, Guo K, Zeng Q, Wu Q, Ng HH, Karsenty G, Crombrugghe BD, Li B (2006) p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol 172:115–125
Miyashita T, Reed JC (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293–299
Antonsson B (2004) Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol Cell Biochem 256-257:141–155
Savitskaya MA, Onishchenko GE (2015) Mechanisms of apoptosis. Biochemistry 80:1393–1405
Johnson VJ, Kim RP, Sharma SH (2005) Aluminum-maltolate induces apoptosis and necrosis in neuro-2a cells: potential role for p53 signaling. Toxicol Sci 83:329–339
Wysokinski D, Pawlowska E, Blasiak J (2015) RUNX2: a master bone growth regulator that may be involved in the DNA damage response. DNA Cell Biology 34:305–315
Artigas N, Gámez B, Cubillosrojas M, Sánchezde DC, Valer JA, Pons G, Rosa JL, Ventura F (2017) p53 inhibits SP7/Osterix activity in the transcriptional program of osteoblast differentiation. Cell Death Differ 24:2022–2031
Vimalraj S, Arumugam B, Miranda PJ, Selvamurugan N (2015) Runx2: structure, function, and phosphorylation in osteoblast differentiation. Int J Biol Macromol 78:202–208
Toshihisa K (2006) Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99:1233–1239
Yang X, Huo H, Xiu C, Song M, Han Y, Li Y, Zhu Y (2016) Inhibition of osteoblast differentiation by aluminum trichloride exposure is associated with inhibition of BMP-2/Smad pathway component expression. Food Chem Toxicol 97:120–126
Funding
This work was supported by the National Natural Science Foundation Project (31872530), the “Young Talent” Project of Northeast Agricultural University (18QC44), the Earmarked Fund For China Agriculture Research System (CARS-35), and the Open Project Program of Northeastern Science Inspection Station, China Ministry of Agriculture Key Laboratory of Animal Pathogen Biology (DY201709).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 402 kb)
Rights and permissions
About this article
Cite this article
Cao, Z., Geng, X., Jiang, X. et al. Melatonin Attenuates AlCl3-Induced Apoptosis and Osteoblastic Differentiation Suppression by Inhibiting Oxidative Stress in MC3T3-E1 Cells. Biol Trace Elem Res 196, 214–222 (2020). https://doi.org/10.1007/s12011-019-01893-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01893-2