Abstract
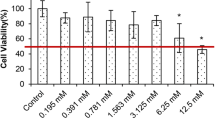
Zinc takes part in several of cellular signaling pathways, containing defense against free radicals, apoptosis, and inflammation. However, interaction between zinc and prostate cancer progression is poorly understood. Therefore, zinc treatment in DU-145 human prostate cancer cells was investigated. First, zinc sulfate (ZnSO4) concentrations with antiproliferative effect were determined using MTT assay. Then, ZnSO4-induced oxidative damage was evaluated by malondialdehyde (MDA) levels, glutathione (GSH) levels, total oxidant status (TOS) levels, and total antioxidant status (TAS) levels. Apoptotic effects of ZnSO4 were determined by measuring biochemical and immunohistochemical parameters including caspase 3 (CASP3), cytochrome C (CYC), Bcl-2-associated X protein (Bax), and B cell CLL/lymphoma 2 (Bcl-2) levels. Inflammatory effects of ZnSO4 were investigated by measuring interleukin-6 (IL-6) levels and tumor necrosis factor-alpha (TNF-α) levels. Finally, morphological analysis was performed using hematoxylin-eosin staining. We found that ZnSO4 caused a concentration-dependent increase in oxidative stress, apoptosis, and inflammation pathways. Moreover, there were a number of morphological alterations in treated cells depending on the ZnSO4 concentration. Consequently, our data showed that zinc acts as a regulator of increased oxidative damage and apoptosis through the upregulation of TNF-α and IL-6.






Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:359–386. https://doi.org/10.1002/ijc.29210
Pernar CH, Ebot EM, Wilson KM, Mucci LA (2018) The epidemiology of prostate cancer. Cold Spring Harb Perspect Med 8:a030361. https://doi.org/10.1101/cshperspect.a030361
Moul JW (2003) Variables in predicting survival based on treating “PSA-only” relapse. Urol Oncol 21:292–304. https://doi.org/10.1016/S1078-1439(03)00103-0
Costell LC, Franklin RB (1998) Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate 35:285–296. https://doi.org/10.1002/(SICI)1097-0045(19980601)35:4<285::AID-PROS8>3.0.CO;2-F
Federico A, Iodice P, Federico P, Del Rio A, Mellone MC, Catalano G (2001) Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur J Clin Nutr 55:293–297. https://doi.org/10.1038/sj.ejcn.1601157
Rohrmann S, Giovannucci E, Willett WC, Platz EA (2007) Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am J Clin Nutr 85:523–529. https://doi.org/10.1093/ajcn/85.2.523
Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, Penson DF, Thompson IM (2008) Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol 167:925–934. https://doi.org/10.1093/aje/kwm389
Costello LC, Franklin RB (2016) A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys 611:100–112. https://doi.org/10.1016/j.abb.2016.04.014
Costello LC, Franklin RB, Feng P, Tan M, Bagasra O (2005) Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States). Cancer Causes Control 16:901–915. https://doi.org/10.1007/s10552-005-2367-y
Hong SH, Choi YS, Cho HJ, Lee JY, Kim JC, Hwang TK, Kim SW (2012) Antiproliferative effects of zinc-citrate compound on hormone refractory prostate cancer. Chin J Cancer Res 24:124–129. https://doi.org/10.1007/s11670-012-0124-9
Feng P, Li TL, Guan ZX, Franklin RB, Costello LC (2003) Effect of zinc on prostatic tumorigenicity in nude mice. Ann N Y Acad Sci 1010:316–320. https://doi.org/10.1196/annals.1299.056
Reyes JG (1996) Zinc transport in mammalian cells. Am J Phys 270:401–410. https://doi.org/10.1152/ajpcell.1996.270.2.C401
McCord MC, Aizenman E (2014) The role of intracellular zinc release in aging, oxidative stress, and Alzheimer's disease. Front Aging Neurosci 6:1–16. https://doi.org/10.3389/fnagi.2014.00077
Liu RY, Fan C, Mitchell S, Chen Q, Wu J, Zuckerman KS (1998) The role of type I and type II tumor necrosis factor (TNF) receptors in the ability of TNF-alpha to transduce a proliferative signal in the human megakaryoblastic leukemic cell line Mo7e. Cancer Res 58:2217–2223
Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M (2016) IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol 11:1482–1492. https://doi.org/10.1016/j.jtho.2016.05.025
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899. https://doi.org/10.1016/j.cell.2010.01.025
Tse BW, Scott KF, Russell PJ (2012) Paradoxical roles of tumour necrosis factor-alpha in prostate cancer biology. Prostate Cancer 2012:1–8. https://doi.org/10.1155/2012/128965
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Srivastava SK, Beutler E (1968) Accurate measurement of oxidized glutathione content of human, rabbit, and rat red blood cells and tissues. Anal Biochem 25:70–76. https://doi.org/10.1016/0003-2697(68)90082-1
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008:pdb.prot4986. https://doi.org/10.1101/pdb.prot4986
Ho E (2004) Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem 15:572–578. https://doi.org/10.1016/j.jnutbio.2004.07.005
Provinciali M, Di Stefano G, Fabris N (1995) Dose-dependent opposite effect of zinc on apoptosis in mouse thymocytes. Int J Immunopharmacol 17:735–744. https://doi.org/10.1016/0192-0561(95)00063-8
Schrantz N, Auffredou MT, Bourgeade MF, Besnault L, Leca G, Vazquez A (2001) Zinc-mediated regulation of caspases activity: dose-dependent inhibition or activation of caspase-3 in the human Burkitt lymphoma B cells (Ramos). Cell Death Differ 8:152–161. https://doi.org/10.1038/sj.cdd.4400772
Wetherell D, Baldwin GS, Shulkes A, Bolton D, Ischia J, Patel O (2018) Zinc ion dyshomeostasis increases resistance of prostate cancer cells to oxidative stress via upregulation of HIF1α. Oncotarget 9:8463–8477. https://doi.org/10.18632/oncotarget.23893
Yan M, Hardin K, Ho E (2010) Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. J Nutr Biochem 21:687–694. https://doi.org/10.1016/j.jnutbio.2009.04.002
Barzilai A, Rotman G, Shiloh Y (2002) ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair 22:3–25. https://doi.org/10.1016/S1568-7864(01)00007-6
Naka K, Muraguchi T, Hoshii T, Hirao A (2008) Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal 10:1883–1894. https://doi.org/10.1089/ars.2008.2114
Sauer H, Wartenberg M, Hescheler J (2001) Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem 11:173–186. https://doi.org/10.1159/000047804
Minelli A, Bellezza I, Conte C, Culig Z (2009) Oxidative stress-related aging: a role for prostate cancer? Biochim Biophys Acta 1795:83–91. https://doi.org/10.1016/j.bbcan.2008.11.001
Bouayed J, Bohn T (2010) Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med Cell Longev 3:228–237. https://doi.org/10.4161/oxim.3.4.12858
Maret W (2013) Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr 4:82–91. https://doi.org/10.3945/an.112.003038
Rudolf E, Rudolf K, Cervinka M (2005) Zinc induced apoptosis in HEP-2 cancer cells: the role of oxidative stress and mitochondria. Biofactors 23:107–120. https://doi.org/10.1002/biof.5520230206
Nagata S (2018) Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36:489–517. https://doi.org/10.1146/annurev-immunol-042617-053010
Elmore S (2007) Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59. https://doi.org/10.1038/nrm2308
Costello LC, Feng P, Milon B, Tan M, Franklin RB (2004) Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis 7:111–117. https://doi.org/10.1038/sj.pcan.4500712
Michalaki V, Syrigos K, Charles P, Waxman J (2004) Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 90:2312–2316. https://doi.org/10.1038/sj.bjc.6601814
Muenchen HJ, Lin DL, Walsh MA, Keller ET, Pienta KJ (2000) Tumor necrosis factor-alpha-induced apoptosis in prostate cancer cells through inhibition of nuclear factor-kappaB by an IkappaBalpha “super-repressor”. Clin Cancer Res 6:1969–1977
Giri D, Ozen M, Ittmann M (2001) Interleukin-6 is an autocrine growth factor in human prostate cancer. The Am J Pathol 159:2159–2165. https://doi.org/10.1016/S0002-9440(10)63067-2
Maolake A, Izumi K, Natsagdorj A, Iwamoto H, Kadomoto S, Makino T, Naito R, Shigehara K, Kadono Y, Hiratsuka K, Wufuer G, Nastiuk KL, Mizokami A (2018) Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR7 upregulation. Cancer Sci 109:1524–1531. https://doi.org/10.1111/cas.13586
Lu L, Shi W, Deshmukh RR, Long J, Cheng X, Ji W, Zeng G, Chen X, Zhang Y, Dou QP (2014) Tumor necrosis factor-α sensitizes breast cancer cells to natural products with proteasome-inhibitory activity leading to apoptosis. PLoS One 9:e113783. https://doi.org/10.1371/journal.pone.0113783
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hacioglu, C., Kacar, S., Kar, F. et al. Concentration-Dependent Effects of Zinc Sulfate on DU-145 Human Prostate Cancer Cell Line: Oxidative, Apoptotic, Inflammatory, and Morphological Analyzes. Biol Trace Elem Res 195, 436–444 (2020). https://doi.org/10.1007/s12011-019-01879-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01879-0