Abstract
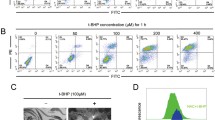
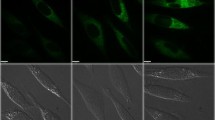
Iron accumulation plays a major role in neuronal cell death which has severe effects on mental health like neurodegenerative disorders. The present work aims to explore the involvement of molecular pathways involved in iron-mediated neuronal cell death using Ferric Ammonium Citrate (FAC) as a source of iron to treat neuroblastoma SH-SY5Y cells. In this study, it was found that cytotoxicity induced by iron treatment is highly correlated with enhanced intracellular reactive oxygen species (ROS) generation and loss of mitochondrial integrity. Appearance of early and late apoptotic cells with altered nuclear morphology and increased expression of effector proteins, i.e., cleaved Caspase 3 and cleaved PARP (Poly-ADP-ribose Polymerase), clearly confirmed iron-induced apoptotic cell deaths. Furthermore, excess accumulation of acidic vesicles and microtubule-associated protein 1 light chain 3 (LC3) puncta and LC3II/I expressions were observed. Simultaneously, ultrastructural studies of SH-SY5Y cells demonstrated the accumulation of a large number of autophagosomes, autophagic vacuolization, and swollen mitochondria which further confirmed the induction of autophagy concomitant with mitochondrial damage. Furthermore, increased incorporation of lysosome-specific dye, LysoTracker Deep Red, and the red fluorescence retention of LC3-GFP-RFP constructs indicates the incomplete autophagy or autophagy dysfunction due to altered lysosomal activity. Hence, the present work unveiled the interruption in autophagy progression caused by the plausible suppression of lysosomal activity due to iron treatment resulting in autophagic cell death in SH-SY5Y cell lines. In general, both apoptotic and autophagic pathways were prominent and each of the pathways played their prospective roles, in iron-mediated neuronal cell death.










Similar content being viewed by others
References
Jaeger AP, Wyss-Coray T (2009) All-you-can-eat: autophagy in neurodegeneration and neuroprotection. Mol Neurodegener 4:16. https://doi.org/10.1186/1750-1326-4-16
Zhang Z, Miah M, Culbreth M, Aschner M (2016) Autophagy in neurodegenerative diseases and metal neurotoxicity. Neurochem Res 41:409–422. https://doi.org/10.1007/s11064-016-1844-x
Martinez-Vicente M (2015) Autophagy in neurodegenerative diseases: from pathogenic dysfunction to therapeutic modulation. Semin Cell Dev Biol 40:115–126. https://doi.org/10.1016/j.semcdb.2015.03.005
Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E (2009) Beclin1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parinson’s and Lewy body diseases. J Neurosci 29(43):13578–13588. https://doi.org/10.1523/JNEUROSCI.4390-09.2009.
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10:9–17. https://doi.org/10.1038/nchembio.1416
Salvador GA (2010) Iron in neuronal function and dysfunction. BioFactors 36:103–110. https://doi.org/10.1002/biof.80
Wu Y, Li X, Xie W, Jankovic J, Le W, Pan T (2010) Neuroprotection of deferoxamine on rotenone – induced injury via accumulation of HIF-1α and induction of autophagy in SH-Y5Y cells. Neurochem Int 57:198–205. https://doi.org/10.1016/j.neuint.2010.05.008
Chen CW, Chen TY, Tsai KL, Lin CL, Yokoyama KK, Lee WS, Chiueh CC, Hsu C (2012) Inhibition of autophagy as a therapeutic strategy of iron-induced brain injury after hemorrhage. Autophagy 8:1510–1520. https://doi.org/10.4161/auto.21289
Castino R, Fiorentio I, Cagnin M, Giovia A, Isidoro C (2011) Chelation of lysosomal iron protects dopaminergic SH-SY5Y neuroblastoma cells from hydrogen peroxide toxicity by precluding autophagy and AKT dephosphorylation. Toxicol Sci 123:523–541. https://doi.org/10.1093/toxsci/kfr179
Kidane TZ, Sauble E, Linder MC (2006) Release of iron from ferritin requires lysosomal activity. Am J Phys Cell Physiol 291:C445–C455. https://doi.org/10.1152/ajpcell.00505.2005
Guo F, Liu X, Cai H, Le W (2017) Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol 28:3–13. https://doi.org/10.1111/bpa.12545
Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J (2008) Body iron metabolism and pathophysiology of iron overload. Int J Hematol 88:7–15. https://doi.org/10.1007/s12185-008-0120-5
Hare D, Ayton S, Bush A, Lei P (2013) A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci 5:34. https://doi.org/10.3389/fnagi.2013.00034
Wan W, Jin L, Wang Z, Wang L, Fei G, Ye F, Pan X, Wang C, Zhong C (2017) Iron deposition leads to neuronal α-synuclein pathology by inducing autophagy dysfunction. Front Neurol 8:1. https://doi.org/10.3389/fneur.2017.00001
Nunez MT, Munoz P, Tapia V, Esparza A, Salazar J, Speisky H (2004) Progressive iron accumulation induces a biphasic change in the glutathione content of neuroblastoma cells. Free Radic Biol Med 37:953–960. https://doi.org/10.1016/j.freeradbiomed.2004.06.005
Wadia JS, Chalmers-Redman RME, Ju WJH, Carlile GW, Phillips JL, Fraser AD, Tatton WG (1998) Mitochondrial membrane potential and nuclear changes in apoptosis caused by serum and nerve growth factor withdrawal: time course and modification by (−)-deprenyl. J Neurosci 18:932–947. https://doi.org/10.1523/JNEUROSCI.18-03-00932.1998
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: iron-dependent form of non-apoptotic cell death. Cell 149:1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Crowley LC, Marfell BJ, Waterhouse NJ (2016) Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb Protoc 2016(9):pdb.prot087205. https://doi.org/10.1101/pdb.prot087205
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104. https://doi.org/10.1038/sj.cdd.4400476
Chaitanya GV, Steven AJ, Babu PP (2010) PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 8:31. https://doi.org/10.1186/1478-811X-8-31
Kroemer G, Levine B (2008) Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9:1004–1010. https://doi.org/10.1038/nrm2529
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12. https://doi.org/10.1002/path.2697
Tanida I, Waguri S (2010) Measurement of autophagy in cells and tissues. Methods Mol Biol 648:193–214. https://doi.org/10.1007/978-1-60761-756-3_13
Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E (2005) Lysosomal turnover, but not a cellular level, of endogeneous LC3 is a marker for autophagy. Autophagy 1:84–91
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, Coppes RP, Engedal N, Mari M, Reggiori F (2018) Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosomefusion. Autophagy. 14:1435–1455. https://doi.org/10.1080/15548627.2018.1474314
Kroemer G, Levine B (2008) Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9(12):1004–1010. https://doi.org/10.1038/nrm2527
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111. https://doi.org/10.1091/mbc.e03-09-0704
Pivtoraiko VN, Stone SL, Roth KA, Shacka JJ (2009) Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. Antioxid Redox Signal 11:481–496. https://doi.org/10.1089/ARS.2008.2263
Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA, Koh JY (2010) Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Invest Ophthalmol Vis Sci 51:6030–6037. https://doi.org/10.1167/iovs.10-5278
Hansen TE, Johansen T (2011) Following autophagy step by step. BMC Biol 20119:39. https://doi.org/10.1186/1741-7007-9-39
Tanida I, Ueno T, Kominami E (2008) LC3 and autophagy. Methods Mol Biol 445:77–88. https://doi.org/10.1159/000478626
Das G, Shravage BV, Baehrecke EH (2012) Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol 4:a008813. https://doi.org/10.1101/cshperspect.a008813
Acknowledgments
JB gratefully acknowledges the financial support from the University Grants Commission (Major Research Project) No. 41-539/2012 (SR) and West Bengal University of Technology TEQIP II program for providing infrastructural facilities. JR is further thankful to ICMR SRF (No. 46/68/2015/BMS/TRM) for providing Senior Research Fellowship. SR acknowledges SERB for awarding the National Post Doctoral Fellowship (PDF/2017/001443). The authors are also thankful to Bose Institute, Kolkata, for providing the flow cytometry facilities and National Institute of Cholera and Enteric Diseases, Kolkata, for providing the TEM facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rakshit, J., Mallick, A., Roy, S. et al. Iron-Induced Apoptotic Cell Death and Autophagy Dysfunction in Human Neuroblastoma Cell Line SH-SY5Y. Biol Trace Elem Res 193, 138–151 (2020). https://doi.org/10.1007/s12011-019-01679-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01679-6