Abstract
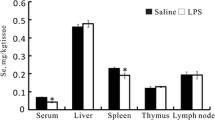
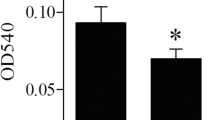
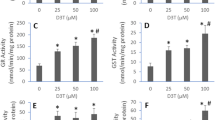
This study was conducted to profile selenoprotein encoding genes in mouse RAW264.7 cells upon lipopolysaccharide (LPS) challenge and integrate their roles into immunological regulation in response to selenium (Se) pretreatment. LPS was used to develop immunological stress in macrophages. Cells were pretreated with different levels of Se (0, 0.5, 1.0, 1.5, 2.0 μmol Se/L) for 2 h, followed by LPS (100 ng/mL) stimulation for another 3 h. The mRNA expression of 24 selenoprotein encoding genes and 9 inflammation-related genes were investigated. The results showed that LPS (100 ng/mL) effectively induced immunological stress in RAW264.7 cells with induced inflammation cytokines, IL-6 and TNF-α, mRNA expression, and cellular secretion. LPS increased (P < 0.05) mRNA profiles of 9 inflammation-related genes in cells, while short-time Se pretreatment modestly reversed (P < 0.05) the LPS-induced upregulation of 7 genes (COX-2, ICAM-1, IL-1β, IL-6, IL-10, iNOS, and MCP-1) and further increased (P < 0.05) expression of IFN-β and TNF-α in stressed cells. Meanwhile, LPS decreased (P < 0.05) mRNA levels of 18 selenoprotein encoding genes and upregulated mRNA levels of TXNRD1 and TXNRD3 in cells. Se pretreatment recovered (P < 0.05) expression of 3 selenoprotein encoding genes (GPX1, SELENOH, and SELENOW) in a dose-dependent manner and increased (P < 0.05) expression of another 5 selenoprotein encoding genes (SELENOK, SELENOM, SELENOS, SELENOT, and TXNRD2) only at a high level (2.0 μmol Se/L). Taken together, LPS-induced immunological stress in RAW264.7 cells accompanied with the global downregulation of selenoprotein encoding genes and Se pretreatment alleviated immunological stress via upregulation of a subset of selenoprotein encoding genes.




Similar content being viewed by others
References
Glaros T, Larsen M, Li L (2009) Macrophages and fibroblasts during inflammation, tissue damage and organ injury. Front Biosci 14(10):3988–3993
Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C (2003) IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 24(1):25–29
Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE (2007) Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clinical & Experimental Immunology 147(2):227–235
Yoon WJ, Ham YM, Yoo BS, Moon JY, Koh J, Hyun CG (2009) Oenothera laciniata inhibits lipopolysaccharide induced production of nitric oxide, prostaglandin E2, and proinflammatory cytokines in RAW264.7 macrophages. J Biosci Bioeng 107(4):429–438
Lu CL, Wei Z, Min W, Hu MM, Chen WL, Xu XJ, Lu CJ (2015) Polysaccharides from smilax glabra inhibit the pro-inflammatory mediators via ERK1/2 and JNK pathways in LPS-induced RAW264.7 cells. Carbohydr Polym 122:428–436
Lee HJ, Shin JS, Lee WS, Shim HY, Park JM, Jang DS, Lee KT (2016) Chikusetsusaponin IVa methyl ester isolated from the roots of achyranthes japonica suppresses LPS-induced iNOS, TNF-ɑ, IL-6, and IL-1β expression by NF-κB and AP-1 inactivation. Biol Pharm Bull 39(5):657–664
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Laskin DL, Pendino KJ (1995) Macrophages and inflammatory mediators in tissue injury. Annual Review of Pharmacology & Toxicology 35(1):655–677
Macmicking J, Xie Q, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15(15):323–350
Davis KL, Martin E, Turko IV, Murad F (2001) Novel effects of nitric oxide. Annual Review of Pharmacology & Toxicology 41(1):203–236
Rhule A, Navarro S, Smith JR, Shepherd DM (2006) Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. J Ethnopharmacol 106(1):121–128
Abarikwu SO (2014) Anti-inflammatory effects of kolaviron modulate the expressions of inflammatory marker genes, inhibit transcription factors ERK1/2, p-JNK, NF-κB, and activate Akt expressions in the 93RS2 Sertoli cell lines. Mol Cell Biochem 401(1):1–12
Park SY, Seetharaman R, Ko MJ, Kim DY, Kim TH, Yoon MK, Kwak JH, Lee SJ, Bae YS, Choi YW (2014) Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int Immunopharmacol 19(2):253–261
Xu XL, Yin P, Wan CR, Chong XL, Liu MJ, Cheng P, Chen JJ, Liu FH, Xu JQ (2014) Punicalagin inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation 37(3):956–965
Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK (2006) Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem 281(44):33019–33029
Kim JY, Park SJ, Yun KJ, Cho YW, Park HJ, Lee KT (2008) Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW264.7 macrophages. Eur J Pharmacol 584(1):175–184
Li L, Sapkota M, Kim SW, Soh Y (2015) Herbacetin inhibits inducible nitric oxide synthase via JNK and nuclear factor-κB in LPS-stimulated RAW264.7 cells. Eur J Pharmacol 765:115–123
Jung HW, Mahesh R, Park JH, Boo YC, Park KM, Park YK (2010a) Bisabolangelone isolated from ostericum koreanum inhibits the production of inflammatory mediators by down-regulation of NF-κB and ERK MAP kinase activity in LPS-stimulated RAW264.7 cells. Int Immunopharmacol 10(2):155–162
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11):1273–1280
Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16(7):705–743
Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G (1992) Regulation of cellular immune responses by selenium. Biol Trace Elem Res 33(1–3):23–35
Hawkes WC, Alkan Z (2010) Regulation of redox signaling by selenoproteins. Biol Trace Elem Res 134(3):235–251
Khoso PA, Yang Z, Liu C, Li S (2015) Selenium deficiency downregulates selenoproteins and suppresses immune function in chicken thymus. Biol Trace Elem Res 167(1):48–55
Zhang ZW, Wang QH, Zhang JL, Li S, Wang XL, Xu SW (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149(3):352–361
Stoedter M, Renko K, Hög A, Schomburg L (2010) Selenium controls the sex-specific immune response and selenoprotein expression during the acute-phase response in mice. Biochem J 429(1):43–51
Zhang W, Zhang R, Wang T, Jiang H, Guo M, Zhou E, Sun Y, Yang Z, Xu S, Cao Y (2014) Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation 37(2):478–485
Kim SH, Johnson VJ, Shin TY, Sharma RP (2004) Selenium attenuates lipopolysaccharide-induced oxidative stress responses through modulation of p38 MAPK and NF-κB signaling pathways. Experimental Biology & Medicine 229(2):203–213
Lobanov AV, Hatfield DL, Gladyshev VN (2009) Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta 1790(11):1424–1428
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54
Stadtman TC (2000) Selenium biochemistry: mammalian selenoenzymes. Ann N Y Acad Sci 899(1):399–402
Carlson BA, Min-Hyuk Y, Yasuyo S, Aniruddha S, Young KJ, Robert I, Gladyshev VN, Hatfield DL, Mo PJ (2009) Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression. BMC Immunol 10(1):1–12
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Tang JY, Huang XF, Wang LQ, Li Q, Xu JY, Jia G, Liu GM, Chen XL, Shang HY, Zhao H (2016) Supranutritional dietary selenium depressed expression of selenoprotein genes in three immune organs of broilers. Anim Sci J 88(2):331–338
Xu JY, Wang LQ, Tang JY, Jia G, Liu GM, Chen XL, Cai JY, Shang HY, Zhao H (2017) Pancreatic atrophy caused by dietary selenium deficiency induces hypoinsulinemic hyperglycemia via global down-regulation of selenoprotein encoding genes in broilers. PLoS One 12(8):e0182079
Vo VA, Lee JW, Shin SY, Kwon JH, Lee HJ, Kim SS, Kwon YS, Chun W (2014) Methyl p-Hydroxycinnamate suppresses lipopolysaccharide-induced inflammatory responses through Akt phosphorylation in RAW264.7 cells. Biomol Ther 22(1):10–16
Jung HW, Mahesh R, Lee JG, Lee SH, Kim YS, Park YK (2010b) Pinoresinol from the fruits of forsythia koreana inhibits inflammatory responses in LPS-activated microglia. Neurosci Lett 480(3):215–220
Kim YS, Ahn CB, Je JY (2016) Anti-inflammatory action of high molecular weight mytilus edulis hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-κB and MAPK pathways. Food Chem 202:9–14
Gu CP, Yu FL, Yu L, He XY, Zhong DS, He LG, Lv LY, Xie L, Liu SW (2014) A novel synthetic dibenzocyclooctadiene lignan analog XLYF-104-6 attenuates lipopolysaccharide-induced inflammatory response in RAW264.7 macrophage cells and protects BALB/c mice from sepsis. Eur J Pharmacol 729(1):22–29
Yun KJ, Kim JY, Kim JB, Lee KW, Jeong SY, Park HJ, Jung HJ, Cho YW, Yun K, Lee KT (2008) Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappaB inactivation in RAW264.7 macrophages: possible involvement of the IKK and MAPK pathways. Int Immunopharmacol 8(3):431–441
Ma JS, Kim WJ, Kim JJ, Kim TJ, Ye SK, Song MD, Kang H, Kim DW, Moon WK, Lee KH (2010) Gold nanoparticles attenuate LPS-induced NO production through the inhibition of NF-kappaB and IFN-beta/STAT1 pathways in RAW264.7 cells. Nitric Oxide Biology & Chemistry 23(3):214–219
Wang YR, Cui YT, Cao FY, Qin YY, Li WJ, Zhang JH (2015) Ganglioside GD1a suppresses LPS-induced pro-inflammatory cytokines in RAW264.7 macrophages by reducing MAPKs and NF-κB signaling pathways through TLR4. Int Immunopharmacol 28(1):136–145
Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong KM (1998) IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Investig 101(2):311–320
Nguyen TV, Yuan L, Azevedo MS, Jeong KI, Gonzalez AM, Saif LJ (2007) Transfer of maternal cytokines to suckling piglets: in vivo and in vitro models with implications for immunomodulation of neonatal immunity. Veterinary Immunology & Immunopathology 117(3–4):236–248
Wu XF, Ouyang ZJ, Feng LL, Chen G, Guo WJ, Shen Y, Wu XD, Sun Y, Xu Q (2014) Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicology & Applied Pharmacology 281(1):146–156
Ohshima H, Bartsch H (1994) Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 305(2):253–264
Cryer B, Feldman M (1998) Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med 104(5):413–421
Yoon WJ, Ham YM, Yang ET, Kim JY, Kim SS, Yoo BS, Moon JY, Lee WJ, Lee NH, Hyun CG (2009) Suppression of pro-inflammatory cytokines, iNOS, and COX2 expression by brown algae sargassum micracanthum in RAW264.7 macrophages. Eurasian Journal of Biosciences 3(3):130–143
Nishishiro M, Kurihara T, Wakabayashi H, Sakagami H (2009) Effect of tropolone, azulene and azulenequinone derivatives on prostaglandin E2 production by activated macrophage-like cells. Anticancer Res 29(1):379–383
Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, Denissenko MF (2005) Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis 26(5):943–950
Hubbard AK, Rothlein R (2000) Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med 28(9):1379–1386
Beutler B (2003) Science review: key inflammatory and stress pathways in critical illness-the central role of the toll-like receptors. Crit Care 7(1):39–46
Samavati L, Rastogi R, Du W, Hüttemann M, Fite A, Franchi L (2009) STAT3 tyrosine phosphorylation is critical for interleukin 1beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol 46(8–9):1867–1877
Kim YS, Ko HM, Kang NI, Song CH, Zhang X, Chung WC, Kim JH, Choi IH, Park YM, Kim GY (2007) Mast cells play a key role in the developmentof late airway hyperresponsiveness through TNF-ɑ in a murine model of asthma. Eur J Immunol 37(4):1107–1115
Simsek I (2010) TNF inhibitors-new and old agents for rheumatoid arthritis. Bull Nyu Hosp Jt Dis 68(3):204–210
Stokkers PC, Camoglio L, van Deventer SJ (1995) Tumor necrosis factor (TNF) in inflammatory bowel disease: gene polymorphisms, animal models, and potential for anti-TNF therapy. J Inflamm 47(1–2):97–103
Takematsu H, Ozawa H, Yoshimura T, Hara M, Sakakibara A, Oyama J, Tagami H (1991) Systemic TNF administration in psoriatic patients: a promising therapeutic modality for severe psoriasis. Br J Dermatol 124(2):209–210
Cao L, Tang JY, Li Q, Xu JY, Jia G, Liu GM, Chen XL, Shang HY, Cai JY, Zhao H (2016) Expression of selenoprotein genes is affected by heat stress in IPEC-J2 cells. Biol Trace Elem Res 172(2):354–360
Zhao H, Li K, Tang JY, Zhou JC, Wang KN, Xia XJ, Lei XG (2015) Expression of selenoprotein genes is affected by obesity of pigs fed a high-fat diet. J Nutr 145(7):1394–1401
Sun LH, Pi DA, Zhao L, Wang XY, Zhu LY, Qi DS, Liu YL (2017) Response of selenium and selenogenome in immune tissues to LPS-induced inflammatory reactions in pigs. Biol Trace Elem Res 177(1):90–96
Carlson BA, Yoo MH, Shrimali RK, Irons R, Gladyshev VN, Hatfield DL, Park JM (2010) Role of selenium-containing proteins in T-cell and macrophage function. Proc Nutr Soc 69(3):300–310
Zhuang TH, Xu HB, Hao S, Ren F, Chen XX, Pan CL, Huang KH (2015) Effects of selenium on proliferation, interleukin-2 production and selenoprotein mRNA expression of normal and dexamethasone-treated porcine splenocytes. Res Vet Sci 98(Z1):59–65
Yu D, Li JL, Zhang JL, Gao XJ, Xu S (2011) Effects of dietary selenium on selenoprotein W gene expression in the chicken immune organs. Biol Trace Elem Res 144(1–3):678–687
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590
Yao HD, Zhao WC, Zhao X, Fan R, Khoso PA, Zhang Z, Liu W, Xu S (2014) Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol Trace Elem Res 161(3):318–327
Sheridan PA, Zhong N, Carlson BA, Perella CM, Hatfield DL, Beck MA (2007) Decreased selenoprotein expression alters the immune response during influenza virus infection in mice. J Nutr 137(6):1466–1471
Kretz-Remy C, Arrigo AP (2001) Selenium: a key element that controls NF-κB activation and IκBα half life. Biofactors 14(1–4):117–125
Yu D, Zhang ZW, Yao HD, Li S, Xu SW (2015) The role of selenoprotein W in inflammatory injury in chicken immune tissues and cultured splenic lymphocyte. Biometals 28(1):75–87
Barnes KM, Evenson JK, Raines AM, Sunde RA (2009) Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr 139(2):199–206
Du SQ, Zhou J, Jia Y, Huang KX (2010) SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Archives of Biochemistry & Biophysics 502(2):137–143
Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR (2011) Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol 186(4):2127–2137
Ferguson AD, Labunskyy VM, Fomenko DE, Araç D, Chelliah Y, Amezcua CA, Rizo J, Gladyshev VN, Deisenhofer J (2006) NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J Biol Chem 281(6):3536–3543
Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J (2005) Genetic variation in selenoprotein S influences inflammatory response. Nat Genet 37(11):1234–1241
Hansson HA, Rozell B, Stemme S, Engström Y, Thelander L, Holmgren A (1986) Different cellular distribution of thioredoxin and subunit M1 of ribonucleotide reductase in rat tissues. Exp Cell Res 163(2):363–369
Javvadi P, Hertan L, Kosoff R, Datta T, Kolev J, Mick R, Tuttle SW, Koumenis C (2010) Thioredoxin reductase-1 (TxnRd1) mediates curcumin-induced radiosensitization of squamous carcinoma cells. Cancer Res 70(5):1941–1950
Funding
This work was supported partly by the National Natural Science Foundation of China (Nos. 31772643 and 31272468) and by a research funding provided by Sichuan Longda Animal Husbandry Science and Technology Co., Ltd. (No. 2015SCLD001).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 60 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Jing, J., Yan, H. et al. Selenium Pretreatment Alleviated LPS-Induced Immunological Stress Via Upregulation of Several Selenoprotein Encoding Genes in Murine RAW264.7 Cells. Biol Trace Elem Res 186, 505–513 (2018). https://doi.org/10.1007/s12011-018-1333-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1333-y