Abstract
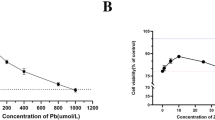
The reproductive system is sensitive to lead (Pb) toxicity, which has long been an area of research interest, but the underlying mechanisms remain to be illustrated. Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) is pivotal in mitochondrial function. In this study, mouse testis Sertoli cells (TM4 cells), PGC-1α lower-expression (PGC-1α(−)) TM4 cells and PGC-1α overexpression (PGC-1α(+)) TM4 cells were used to explore the protective roles of PGC-1α against lead toxicity on the mouse reproductive system. Lead acetate (PbAc) exposure decreased the expression level of PGC-1α, increased the intracellular level of reactive oxygen species (ROS), and reduced the level of ATP in the three TM4 cell lines. The effects of PbAc on intracellular ATP level and on ROS content were significantly weakened in PGC-1α(+)TM4 cells versus TM4 cells and were significantly amplified in PGC-1α(−)TM4 cells versus TM4 cells. These results suggest that PGC-1α is a protective factor against PbAc-induced oxidative stress and energy metabolism dysfunction in the mouse reproductive system, thereby holding the potential of being developed as a preventive or therapeutic strategy against disorders induced by lead exposure.





Similar content being viewed by others
Abbreviations
- PbAc:
-
Lead acetate
- TM4:
-
Mouse testis Sertoli cells
- PGC-1α:
-
Peroxisome proliferator-activated receptor γ coactivator 1α
- ROS:
-
Reactive oxygen species
- LD:
-
Lactic acid
- LDH:
-
Lactic dehydrogenase
- SDH:
-
Succinate dehydrogenase
Reference
Li X, Ye F, Li L, Chang W, Wu X, Chen J (2016) The role of HO-1 in protection against lead-induced neurotoxicity. Neurotoxicology 52:1–11. doi:10.1016/j.neuro.2015.10.015
Ye F, Li X, Li L, Lyu L, Yuan J, Chen J (2015) The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in SH-SY5Y cells. Food Chem Toxicol 86:191–201. doi:10.1016/j.fct.2015.10.009
LH X, FF M, Zhao JH, He Q, Cao CL, Yang H, Liu Q, Liu XH, Sun SJ (2015) Lead induces apoptosis and histone hyperacetylation in rat cardiovascular tissues. PLoS One 10(6):e129091. doi:10.1371/journal.pone.0129091
Bellinger DC (2005) Teratogen update: lead and pregnancy. Birth Defects Res A Clin Mol Teratol 73(6):409–420. doi:10.1002/bdra.20127
Shen W, Chen J, Yin J, Wang SL (2016) Selenium protects reproductive system and foetus development in a rat model of gestational lead exposure. Eur Rev Med Pharmacol Sci 20(4):773–780
Kasperczyk A, Dobrakowski M, Czuba ZP, Horak S, Kasperczyk S (2015) Environmental exposure to lead induces oxidative stress and modulates the function of the antioxidant defense system and the immune system in the semen of males with normal semen profile. Toxicol Appl Pharmacol 284(3):339–344. doi:10.1016/j.taap.2015.03.001
Oktem F, Arslan MK, Dundar B, Delibas N, Gultepe M, Ergurhan II (2004) Renal effects and erythrocyte oxidative stress in long-term low-level lead-exposed adolescent workers in auto repair workshops. Arch Toxicol 78(12):681–687. doi:10.1007/s00204-004-0597-5
Wang ZK, Zhou XL, Song XB, Zhuang DM, Wang ZY, Yang DB, Wang L (2016) Alleviation of lead-induced apoptosis by Puerarin via inhibiting mitochondrial permeability transition pore opening in primary cultures of rat proximal tubular cells. Biol Trace Elem Res. doi:10.1007/s12011-016-0701-8
Hsu PC, Guo YL (2002) Antioxidant nutrients and lead toxicity. Toxicology 180(1):33–44
Li N, Hou YH, Jing WX, Dahms HU, Wang L (2016) Quality decline and oxidative damage in sperm of freshwater crab Sinopotamon henanense exposed to lead. Ecotoxicol Environ Saf 130:193–198. doi:10.1016/j.ecoenv.2016.03.042
Dkhil MA, Moneim AE, Al-Quraishy S (2016) Indigofera Oblongifolia ameliorates lead acetate-induced testicular oxidative damage and apoptosis in a rat model. Biol Trace Elem Res. doi:10.1007/s12011-016-0689-0
Lee NP, Cheng CY (2004) Adaptors, junction dynamics, and spermatogenesis. Biol Reprod 71(2):392–404. doi:10.1095/biolreprod.104.027268
Huang S, Ye J, Yu J, Chen L, Zhou L, Wang H, Li Z, Wang C (2014) The accumulation and efflux of lead partly depend on ATP-dependent efflux pump-multidrug resistance protein 1 and glutathione in testis Sertoli cells. Toxicol Lett 226(3):277–284. doi:10.1016/j.toxlet.2014.02.017
Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y (2010) Sirtuin 3, a new target of PGC-1alpha , plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5(7):e11707. doi:10.1371/journal.pone.0011707
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839
Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105(38):14447–14452. doi:10.1073/pnas.0803790105
Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC (2011) SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell 19(3):416–428. doi:10.1016/j.ccr.2011.02.014
Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR (2005) A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54(7):1926–1933
Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR (2011) Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A 108(35):14608–14613. doi:10.1073/pnas.1111308108
Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y (2010) PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal 13(7):1011–1022. doi:10.1089/ars.2009.2940
Bell EL, Guarente L (2011) The SirT3 divining rod points to oxidative stress. Mol Cell 42(5):561–568. doi:10.1016/j.molcel.2011.05.008
Finkel T, Deng CX, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460(7255):587–591. doi:10.1038/nature08197
Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RJ, Kahn CR, Verdin E (2011) SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 44(2):177–190. doi:10.1016/j.molcel.2011.07.019
Matovic V, Buha A, Ethukic-Cosic D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140. doi:10.1016/j.fct.2015.02.011
Marchlewicz M, Baranowska-Bosiacka I, Kolasa A, Kondarewicz A, Chlubek D, Wiszniewska B (2009) Disturbances of energetic metabolism in rat epididymal epithelial cells as a consequence of chronic lead intoxication. Biometals 22(6):877–887. doi:10.1007/s10534-009-9238-z
Marmolino D, Manto M, Acquaviva F, Vergara P, Ravella A, Monticelli A, Pandolfo M (2010) PGC-1alpha down-regulation affects the antioxidant response in Friedreich’s ataxia. PLoS One 5(4):e10025. doi:10.1371/journal.pone.0010025
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119(9):2758–2771. doi:10.1172/JCI39162
Blanco G, Mercer RW (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Phys 275(5 Pt 2):F633–F650
Zeino M, Brenk R, Gruber L, Zehl M, Urban E, Kopp B, Efferth T (2015) Cytotoxicity of cardiotonic steroids in sensitive and multidrug-resistant leukemia cells and the link with Na(+)/K(+)-ATPase. J Steroid Biochem Mol Biol 150:97–111. doi:10.1016/j.jsbmb.2015.03.008
Mazzitelli LR, Rinaldi DE, Corradi GR, Adamo HP (2010) The plasma membrane Ca2+ pump catalyzes the hydrolysis of ATP at low rate in the absence of Ca2+. Arch Biochem Biophys 495(1):62–66. doi:10.1016/j.abb.2009.12.021
Bhatti JS, Sidhu IP, Bhatti GK (2011) Ameliorative action of melatonin on oxidative damage induced by atrazine toxicity in rat erythrocytes. Mol Cell Biochem 353(1–2):139–149. doi:10.1007/s11010-011-0780-y
Quintanar-Escorza MA, Gonzalez-Martinez MT, Del PI, Calderon-Salinas JV (2010) Oxidative damage increases intracellular free calcium [Ca2+]i concentration in human erythrocytes incubated with lead. Toxicol in Vitro 24(5):1338–1346. doi:10.1016/j.tiv.2010.05.002
Gokulakrishnan A, Ali AR (2010) Cigarette smoke-induced biochemical perturbations in human erythrocytes and attenuation by epigallocatechin-3-gallate—tea catechin. Pharmacol Rep 62(5):891–899
Nava-Ruiz C, Mendez-Armenta M, Rios C (2012) Lead neurotoxicity: effects on brain nitric oxide synthase. J Mol Histol 43(5):553–563. doi:10.1007/s10735-012-9414-2
Baranowska-Bosiacka I, Dziedziejko V, Safranow K, Gutowska I, Marchlewicz M, Dolegowska B, Rac ME, Wiszniewska B, Chlubek D (2009) Inhibition of erythrocyte phosphoribosyltransferases (APRT and HPRT) by Pb2+: a potential mechanism of lead toxicity. Toxicology 259(1–2):77–83. doi:10.1016/j.tox.2009.02.005
Robinson R, Fritz IB (1981) Metabolism of glucose by Sertoli cells in culture. Biol Reprod 24(5):1032–1041
Grootegoed JA, Oonk RB, Jansen R, van der Molen HJ (1986) Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J Reprod Fertil 77(1):109–118
Boussouar F, Benahmed M (2004) Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab 15(7):345–350. doi:10.1016/j.tem.2004.07.003
Jutte NH, Grootegoed JA, Rommerts FF, van der Molen HJ (1981) Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. J Reprod Fertil 62(2):399–405
Acknowledgments
The authors thank all members in Professor CH. Wang’s laboratory in Wuhan University for their valuable assistance and Xiaojie Lu in Tongji University for the revision of the manuscript. The project was supported by The National Natural Science Foundation of China (No.81172628) and Open Fund of Hubei Provincial Key Laboratory for Applied Toxicology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Xi Liu and Jingping Ye contributed equally to this study and share first authorship.
Rights and permissions
About this article
Cite this article
Liu, X., Ye, J., Wang, L. et al. Protective Effects of PGC-1α Against Lead-Induced Oxidative Stress and Energy Metabolism Dysfunction in Testis Sertoli Cells. Biol Trace Elem Res 175, 440–448 (2017). https://doi.org/10.1007/s12011-016-0799-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0799-8