Abstract
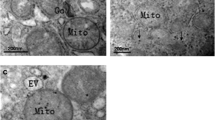
The effects of selenium (Se)-deficient diet on the liver were evaluated by using growing rats which were fed with normal and Se-deficient diets, respectively, for 109 days. The results showed that rats fed with Se-deficient diet led to a decrease in Se concentration in the liver, particularly among male rats from the low-Se group. This causes alterations to the ultrastructure of hepatocytes with condensed chromatin and swelling mitochondria observed after low Se intake. Meanwhile, pathological changes and increased fibrosis in hepatic periportal were detected by hematoxylin and eosin and Masson’s trichrome staining in low-Se group. Furthermore, through immunohistochemistry (IHC) staining, higher expressions of metalloproteinases (MMP1/3) and their tissue inhibitors of metalloproteinases (TIMP1/3) were observed in the hepatic periportal of rats from the low-Se group. However, higher expressions of MMP1/3 and lower expressions of TIMP1/3 were detected in hepatic central vein and hepatic sinusoid. In addition, upregulated expressions of MMP1/3 and downregulated expressions of TIMP1/3 at the messenger RNA (mRNA) and protein levels also appeared to be relevant to low Se intake. In conclusion, Se-deficient diet could cause low Se concentration in the liver, alterations of hepatocyte ultrastructure, differential expressions of MMP1/3 and TIMP1/3 as well as fibrosis in the liver hepatic periportal.





Similar content being viewed by others
References
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6:25–54
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Moghadaszadeh B, Beggs AH (2006) Selenoproteins and their impact on human health through diverse physiological pathways. Physiology 21:307–315
Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw DP (2010) Ecological assessment of selenium in the aquatic environment. CRC Press.
Johnson CC, Fordyce FM, Rayman MP (2010) Symposium on “geographical and geological influences on nutrition”: factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc Nutr Soc 69:119–132
Spallholz JE, Boylan LM, Rhaman MM (2004) Environmental hypothesis: is poor dietary selenium intake an underlying factor for arsenicosis and cancer in Bangladesh and West Bengal, India? Sci Total Environ 323:21–32
Alfthan G, Eurola M, Ekholm P, Venäläinend ER, Rootd T, Korkalainend K, Hartikainenc H, Salminene P, Hietaniemib V, Aspilab P, Aro A (2015) Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. J Trace Elem Med Biol 31:142–147
He S, Guo X, Tan W, Su X, Li J, Wang P, Qiu H (2016) Effect of selenium deficiency on phosphorylation of the AMPK pathway in rats. Biol Trace Elem Res 169:254–260
Han J, Liang H, Yi JH, Tan W, He S, Wu XF, Shi XW, Ma J, Guo X (2016) Selenium deficiency induced damages and altered expressions of metalloproteinases and their inhibitors (MMP1/3, TIMP1/3) in the kidneys of growing rats. J Trace Elem Med Biol 34:1–9
Tan J, Zhu W, Wang W, Li R, Hou S, Wang D, Yang L (2002) Selenium in soil and endemic diseases in China. Sci Total Environ 284:227–235
Patterson BH, Levander OA, Helzlsouer K, McAdam PA, Lewis SA, Taylor PR, Veillon C, Zech LA (1989) Human selenite metabolism: a kinetic model. Am J Physiol-Reg I 257:556–567
Swanson CA, Patterson BH, Levander OA, Veillon C, Taylor PR, Helzlsouer K, McAdam PA, Zech L (1991) Human [74Se] selenomethionine metabolism: a kinetic model. Am J Clin Nutr 54:917–926
Suzuki KT, Ogra Y (2002) Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se. Food Addit Contam 19:974–983
Kenneth JL, Thomas DS (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25:402–408
Kato T, Read R, Rozga J, Burk RF (1992) Evidence for intestinal release of absorbed selenium in a form with high hepatic extraction. Am J Physiol-Gastr L 262:854–858
Hill KE, Wu S, Motley AK, Stevenson TD, Winfrey VP, Capecchi MR, Atkins JF, Burk RF (2012) Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J Biol Chem 287:40414–40424
Han J, Guo X, Lei Y, Dennis BS, Wu SX, Wu CY (2012) Synthesis and characterization of selenium–chondroitin sulfate nanoparticles. Carbohydr Polym 90:122–126
Misra S, Boylan M, Selvam A, Spallholz JE, Björnstedt M (2015) Redox-active selenium compounds from toxicity and cell death to cancer treatment. Nutrition 7:3536–3556
Kohli V, Selzner M, Madden JF, Bentley RC, Clavien PA (1999) Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation 67:1099–1104
Kohli V, Madden JF, Bentley RC, Clavien PA (1999) Calpain mediates ischemic injury of the liver through modulation of apoptosis and necrosis. Gastroenterology 116:168–178
Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H (2000) Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a Caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of Caspase-8. Toxicol Sci 58:109–117
Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE (2007) MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. P Natl Acad Sci USA 104:5318–5323
Li JL, Jiang CY, Li S, Xu SW (2013) Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicol Environ Saf 96:103–109
Wiemerslage L, Lee D (2016) Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J Neurosci Methods 262:56–65
Campbell NA, Williamson B, Heyden RJ (2006) Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall ISBN 0–13–250882-6.
McBride HM, Neuspiel M, Wasiak S (2006) Mitochondria: more than just a powerhouse. Curr Biol 16(14):R551–R560
Liu JT, Guo X, Ma WJ, Zhang YG, Xu P, Yao JF, Bai YD (2010) Mitochondrial function is altered in articular chondrocytes of an endemic osteoarthritis, Kashin-Beck disease. Osteoarthr Cartil 18:1218–1226
Yang F (2006) Keshan disease and mitochondrial cardiomyopathy. Sci China Ser C 49:513–518
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115:209–218
Ramadori G, Saile B (2004) Portal tract fibrogenesis in the liver. Lab Investig 84:153–159
Bataller R, Brenner DA (2001) Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis 21:437–451
Gressner AM (1998) The cell biology of liver fibrogenesis—an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res 292:447–452
Arthur MJP, Iredale JP, Mann DA (1999) Tissue inhibitors of metalloproteinases: role in liver fibrosis and alcoholic liver disease. Alcohol Clin Exp Res 23:940–943
Mott JD, Werb Z (2004) Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16:558–564
Arthur MJP (2000) Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol-Gastr L 279:245–249
Mehdi Y, Hornick JL, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18:3292–3311
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China (81402639, 81472924, 81273008, 81402638) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal experimental procedures followed protocols approved by the Medical Animal Research Ethics Committee at Xi’an Jiaotong University.
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Jing Han and Hua Liang contribute equally to this work.
Rights and permissions
About this article
Cite this article
Han, J., Liang, H., Yi, J. et al. Long-Term Selenium-Deficient Diet Induces Liver Damage by Altering Hepatocyte Ultrastructure and MMP1/3 and TIMP1/3 Expression in Growing Rats. Biol Trace Elem Res 175, 396–404 (2017). https://doi.org/10.1007/s12011-016-0781-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0781-5