Abstract
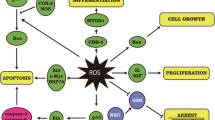
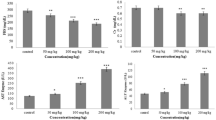
Zinc oxide nanoparticles (ZnONPs) are widely used in food packaging and may enter the body directly if exposed. Hereby, in this study, the oral administration was selected as the route of exposure for rats to nanoparticles and the effect of hesperidin (HSP, 100 mg/kg bwt) was evaluated on ZnONP (600 mg/kg bwt)-induced neurotoxicity in rats. ZnONPs were characterized using transmission electron microscopy. Neurotoxicity was observed as seen by elevation in serum inflammatory markers including tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1β), interleukin-6 (IL-6), C-reactive protein (CRP), and activities of catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione (GSH) content in rat brains. Pretreatment of rats with HSP in ZnONP-treated group elevated activities of antioxidant enzymes. HSP also caused decrease in TNF-α, IL-1β, IL-6, and CRP levels which was higher in the ZnONP-treated group. The results suggest that HSP augments antioxidant defense with anti-inflammatory response against ZnONP-induced neurotoxicity. The increased antioxidant enzymes enhance the antioxidant potential to reduce oxidative stress.




Similar content being viewed by others
References
Gonzalez-Estrella J, Puyol D, Sierra-Alvarez R, Field JA (2015) Role of biogenic sulfide in attenuating zinc oxide and copper nanoparticle toxicity to acetoclastic methanogenesis. J Hazard Mater 283:755–763. doi:10.1016/j.jhazmat.2014.10.030
Zukiene R, Snitka V (2015) Zinc oxide nanoparticle and bovine serum albumin interaction and nanoparticles influence on cytotoxicity in vitro. Colloids Surf B: Biointerfaces 135:316–323. doi:10.1016/j.colsurfb.2015.07.054
Mallevre F, Fernandes TF, Aspray TJ (2014) Silver, zinc oxide and titanium dioxide nanoparticle ecotoxicity to bioluminescent pseudomonas putida in laboratory medium and artificial wastewater. Environ Pollut 195:218–225. doi:10.1016/j.envpol.2014.09.002
Yang X, Jiang MZ (2014) Research progress on biological toxicity of zinc oxide nanoparticle and its mechanism. Zhejiang Da Xue Xue Bao Yi Xue Ban 43(2):218–226
Kao YY, Chen YC, Cheng TJ, Chiung YM, Liu PS (2012) Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol Sci 125(2):462–472. doi:10.1093/toxsci/kfr319
Lee CM, Jeong HJ, Kim DW, Sohn MH, Lim ST (2012) The effect of fluorination of zinc oxide nanoparticles on evaluation of their biodistribution after oral administration. Nanotechnology 23(20):205102. doi:10.1088/0957-4484/23/20/205102
Han D, Tian Y, Zhang T, Ren G, Yang Z (2011) Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int J Nanomedicine 6:1453–1461. doi:10.2147/IJN.S18507
Shim KH, Hulme J, Maeng EH, Kim MK, An SS (2014) Analysis of zinc oxide nanoparticles binding proteins in rat blood and brain homogenate. Int J Nanomedicine 9(Suppl 2):217–224. doi:10.2147/IJN.S58204
Jeng HA, Swanson J (2006) Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health, Part A: Tox Hazard Subst Environ Eng 41(12):2699–2711. doi:10.1080/10934520600966177
Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P (2014) Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int 2014:736385. doi:10.1155/2014/736385
Akhil K, Chandran P, Sudheer Khan S (2015) Influence of humic acid on the stability and bacterial toxicity of zinc oxide nanoparticles in water. J Photochem Photobiol B 153:289–295. doi:10.1016/j.jphotobiol.2015.10.007
Giovanni M, Tay CY, Setyawati MI, Xie J, Ong CN, Fan R, Yue J, Zhang L, Leong DT (2015) Toxicity profiling of water contextual zinc oxide, silver, and titanium dioxide nanoparticles in human oral and gastrointestinal cell systems. Environ Toxicol 30(12):1459–1469. doi:10.1002/tox.22015
Colvin VL (2003) The potential environmental impact of engineered nanomaterials. Nat Biotechnol 21(10):1166–1170. doi:10.1038/nbt875
Danielsen PH, Loft S, Jacobsen NR, Jensen KA, Autrup H, Ravanat JL, Wallin H, Moller P (2010) Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol Sci 118(2):574–585. doi:10.1093/toxsci/kfq290
Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A (2003) Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111(4):455–460
Srivastav AK, Kumar M, Ansari NG, Jain AK, Shankar J, Arjaria N, Jagdale P, Singh D (2016) A comprehensive toxicity study of zinc oxide nanoparticles versus their bulk in Wistar rats: toxicity study of zinc oxide nanoparticles. Hum Exp Toxicol. doi:10.1177/0960327116629530
Ko JW, Hong ET, Lee IC, Park SH, Park JI, Seong NW, Hong JS, Yun HI, Kim JC (2015) Evaluation of 2-week repeated oral dose toxicity of 100 nm zinc oxide nanoparticles in rats. Lab Anim Res 31(3):139–147. doi:10.5625/lar.2015.31.3.139
Choi J, Kim H, Kim P, Jo E, Kim HM, Lee MY, Jin SM, Park K (2015) Toxicity of zinc oxide nanoparticles in rats treated by two different routes: single intravenous injection and single oral administration. J Toxic Environ Health A 78(4):226–243. doi:10.1080/15287394.2014.949949
Hosseinimehr SJ, Jalayer Z, Naghshvar F, Mahmoudzadeh A (2012) Hesperidin inhibits cyclophosphamide-induced tumor growth delay in mice. Integr Cancer Ther 11(3):251–256. doi:10.1177/1534735412448959
Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM (2012) Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complicat 26(6):483–490. doi:10.1016/j.jdiacomp.2012.06.001
Koyuncu H, Berkarda B, Baykut F, Soybir G, Alatli C, Gul H, Altun M (1999) Preventive effect of hesperidin against inflammation in CD-1 mouse skin caused by tumor promoter. Anticancer Res 19(4B):3237–3241
Crespo ME, Galvez J, Cruz T, Ocete MA, Zarzuelo A (1999) Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med 65(7):651–653. doi:10.1055/s-2006-960838
Zhang B, Chen T, Chen Z, Wang M, Zheng D, Wu J, Jiang X, Li X (2012) Synthesis and anti-hyperglycemic activity of hesperidin derivatives. Bioorg Med Chem Lett 22(23):7194–7197. doi:10.1016/j.bmcl.2012.09.049
Hewage SR, Piao MJ, Kang KA, Ryu YS, Han X, Oh MC, Jung U, Kim IG, Hyun JW (2016) Hesperidin attenuates ultraviolet B-induced apoptosis by mitigating oxidative stress in human keratinocytes. Biomol Ther (Seoul). doi:10.4062/biomolther.2015.139
Ohara T, Muroyama K, Yamamoto Y, Murosaki S (2016) Oral intake of a combination of glucosyl hesperidin and caffeine elicits an anti-obesity effect in healthy, moderately obese subjects: a randomized double-blind placebo-controlled trial. Nutr J 15(1):6. doi:10.1186/s12937-016-0123-7
Oguzturk H, Ciftci O, Cetin A, Kaya K, Disli OM, Turtay MG, Gurbuz S, Basak N (2016) Beneficial effects of hesperidin following cis-diamminedichloroplatinum-induced damage in heart of rats. Niger J Clin Pract 19(1):99–103. doi:10.4103/1119-3077.173707
Ahmadi A, Shadboorestan A (2016) Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr Cancer 68(1):29–39. doi:10.1080/01635581.2015.1078822
Polat N, Ciftci O, Cetin A, Yilmaz T (2016) Toxic effects of systemic cisplatin on rat eyes and the protective effect of hesperidin against this toxicity. Cutan Ocul Toxicol 35(1):1–7. doi:10.3109/15569527.2014.999080
Kaur G, Tirkey N, Chopra K (2006) Beneficial effect of hesperidin on lipopolysaccharide-induced hepatotoxicity. Toxicology 226(2–3):152–160. doi:10.1016/j.tox.2006.06.018
Pari L, Shagirtha K (2012) Hesperetin protects against oxidative stress related hepatic dysfunction by cadmium in rats. Exp Toxicol Pathol 64(5):513–520. doi:10.1016/j.etp.2010.11.007
Ahmad ST, Arjumand W, Nafees S, Seth A, Ali N, Rashid S, Sultana S (2012) Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol Lett 208(2):149–161. doi:10.1016/j.toxlet.2011.10.023
Yeh YH, Hsieh YL, Lee YT (2013) Effects of yam peel extract against carbon tetrachloride-induced hepatotoxicity in rats. J Agric Food Chem 61(30):7387–7396. doi:10.1021/jf401864y
Derrell C (1996) Guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources. National Academy Press, Washington, D.C.
Faddah LM, Abdel Baky NA, Al-Rasheed NM, Al-Rasheed NM, Fatani AJ, Atteya M (2012) Role of quercetin and arginine in ameliorating nano zinc oxide-induced nephrotoxicity in rats. BMC Complement Altern Med 12:60. doi:10.1186/1472-6882-12-60
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Factor VM, Kiss A, Woitach JT, Wirth PJ, Thorgeirsson SS (1998) Disruption of redox homeostasis in the transforming growth factor-alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J Biol Chem 273(25):15846–15853
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Muller KH, Kulkarni J, Motskin M, Goode A, Winship P, Skepper JN, Ryan MP, Porter AE (2010) pH-dependent toxicity of high aspect ratio ZnO nanowires in macrophages due to intracellular dissolution. ACS Nano 4(11):6767–6779. doi:10.1021/nn101192z
Hanley C, Layne J, Punnoose A, Reddy KM, Coombs I, Coombs A, Feris K, Wingett D (2008) Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 19(29):295103. doi:10.1088/0957-4484/19/29/295103
Vandebriel RJ, De Jong WH (2012) A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl 5:61–71. doi:10.2147/NSA.S23932
Cho WS, Duffin R, Howie SE, Scotton CJ, Wallace WA, Macnee W, Bradley M, Megson IL, Donaldson K (2011) Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part Fibre Toxicol 8:27. doi:10.1186/1743-8977-8-27
Cho WS, Duffin R, Poland CA, Duschl A, Oostingh GJ, Macnee W, Bradley M, Megson IL, Donaldson K (2012) Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 6(1):22–35. doi:10.3109/17435390.2011.552810
Li CH, Liao PL, Shyu MK, Liu CW, Kao CC, Huang SH, Cheng YW, Kang JJ (2012) Zinc oxide nanoparticles-induced intercellular adhesion molecule 1 expression requires Rac1/Cdc42, mixed lineage kinase 3, and c-Jun N-terminal kinase activation in endothelial cells. Toxicol Sci 126(1):162–172. doi:10.1093/toxsci/kfr331
Li CH, Shen CC, Cheng YW, Huang SH, Wu CC, Kao CC, Liao JW, Kang JJ (2012) Organ biodistribution, clearance, and genotoxicity of orally administered zinc oxide nanoparticles in mice. Nanotoxicology 6(7):746–756. doi:10.3109/17435390.2011.620717
Roy R, Tripathi A, Das M, Dwivedi PD (2011) Cytotoxicity and uptake of zinc oxide nanoparticles leading to enhanced inflammatory cytokines levels in murine macrophages: comparison with bulk zinc oxide. J Biomed Nanotechnol 7(1):110–111
Hsiao IL, Huang YJ (2011) Effects of various physicochemical characteristics on the toxicities of ZnO and TiO nanoparticles toward human lung epithelial cells. Sci Total Environ 409(7):1219–1228. doi:10.1016/j.scitotenv.2010.12.033
Garg A, Garg S, Zaneveld LJ, Singla AK (2001) Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res 15(8):655–669
Omar HA, Mohamed WR, Arafa el SA, Shehata BA, Sherbiny GA, Arab HH, Elgendy AN (2016) Hesperidin alleviates cisplatin-induced hepatotoxicity in rats without inhibiting its antitumor activity. Pharmacol Rep 68(2):349–356. doi:10.1016/j.pharep.2015.09.007
Justin Thenmozhi A, Raja TR, Janakiraman U, Manivasagam T (2015) Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem Res 40(4):767–776. doi:10.1007/s11064-015-1525-1
Iranshahi M, Rezaee R, Parhiz H, Roohbakhsh A, Soltani F (2015) Protective effects of flavonoids against microbes and toxins: the cases of hesperidin and hesperetin. Life Sci 137:125–132. doi:10.1016/j.lfs.2015.07.014
Lee HJ, Lee WJ, Chang SE, Lee GY (2015) Hesperidin, a popular antioxidant inhibits melanogenesis via Erk1/2 mediated MITF degradation. Int J Mol Sci 16(8):18384–18395. doi:10.3390/ijms160818384
Kim GD (2015) Hesperidin inhibits vascular formation by blocking the AKT/mTOR signaling pathways. Prev Nutr Food Sci 20(4):221–229. doi:10.3746/pnf.2015.20.4.221
Khedr NF, Khalil RM (2015) Effect of hesperidin on mice bearing Ehrlich solid carcinoma maintained on doxorubicin. Tumour Biol 36(12):9267–9275. doi:10.1007/s13277-015-3655-0
Khan MH, Parvez S (2015) Hesperidin ameliorates heavy metal induced toxicity mediated by oxidative stress in brain of Wistar rats. J Trace Elem Med Biol 31:53–60. doi:10.1016/j.jtemb.2015.03.002
Kaya K, Ciftci O, Cetin A, Dogan H, Basak N (2015) Hesperidin protects testicular and spermatological damages induced by cisplatin in rats. Andrologia 47(7):793–800. doi:10.1111/and.12332
Martinez RM, Pinho-Ribeiro FA, Steffen VS, Caviglione CV, Vignoli JA, Baracat MM, Georgetti SR, Verri WA Jr, Casagrande R (2015) Hesperidin methyl chalcone inhibits oxidative stress and inflammation in a mouse model of ultraviolet B irradiation-induced skin damage. J Photochem Photobiol B 148:145–153. doi:10.1016/j.jphotobiol.2015.03.030
Tsou TC, Yeh SC, Tsai FY, Lin HJ, Cheng TJ, Chao HR, Tai LA (2010) Zinc oxide particles induce inflammatory responses in vascular endothelial cells via NF-kappaB signaling. J Hazard Mater 183(1–3):182–188. doi:10.1016/j.jhazmat.2010.07.010
Fine JM, Gordon T, Chen LC, Kinney P, Falcone G, Beckett WS (1997) Metal fume fever: characterization of clinical and plasma IL-6 responses in controlled human exposures to zinc oxide fume at and below the threshold limit value. J Occup Environ Med 39(8):722–726
Deng X, Luan Q, Chen W, Wang Y, Wu M, Zhang H, Jiao Z (2009) Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 20(11):115101. doi:10.1088/0957-4484/20/11/115101
Liu XX, Yu DD, Chen MJ, Sun T, Li G, Huang WJ, Nie H, Wang C, Zhang YX, Gong Q, Ren BX (2015) Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. Int Immunopharmacol 25(2):370–376. doi:10.1016/j.intimp.2015.02.022
Kim NH, Oh MK, Park HJ, Kim IS (2010) Auranofin, a gold(I)-containing antirheumatic compound, activates Keap1/Nrf2 signaling via Rac1/iNOS signal and mitogen-activated protein kinase activation. J Pharmacol Sci 113(3):246–254
Acknowledgments
This research project was supported by the “Research Center of the Center for Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia. Also, we would like to thank Ms. Jennifer Hudson and Ms. Sarah Paulson for their help in completion of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ansar, S., Abudawood, M., Hamed, S.S. et al. Exposure to Zinc Oxide Nanoparticles Induces Neurotoxicity and Proinflammatory Response: Amelioration by Hesperidin. Biol Trace Elem Res 175, 360–366 (2017). https://doi.org/10.1007/s12011-016-0770-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0770-8