Abstract
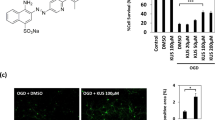
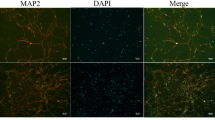
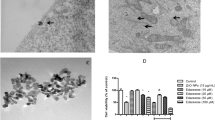
Increased concentration of manganese (Mn) in the brain is known to be associated with excitotoxicity and neuroinflammation. Vinpocetine, an alkaloid derived from the plant Vinca minor L., basically shows its effect via phosphodiesterase inhibition and voltage-dependent Na+ channels. Vasoactive intestinal peptide (VIP) has gastrointestinal, vasomotor, muscular, and neuroprotective effects. The aim of this study was to examine the potential protective effects of vinpocetine and VIP against Mn toxicity in NE-4C neural stem cells (NSCs). VIP treatment at 1 μM and vinpocetine treatment at 2 μM concentrations were sufficient to yield maximum protection, and these concentrations were adopted in the following experiments. In this study, Mn treatment significantly increased lactate dehydrogenase (LDH) leakage, reactive oxygen species (ROS) production, and triggered cell death in NE-4C cultures. However, significant reduction in LDH release was observed following vinpocetine or VIP treatments when compared with control. Similar to these findings, vinpocetine or VIP treatments significantly reduced membrane degradation induced by Mn (p < 0.001). Moreover, vinpocetine attenuated Mn-induced decrease of mitochondrial membrane potential. Similarly, proapoptotic protein bax and ROS production significantly decreased in cells after incubation with vinpocetine (p = 0.01) or VIP in the presence of Mn (p < 0.001). Our study provides the evidence that both vinpocetine and VIP may exert protective effects via modulating oxidative stress and apoptosis in Mn-induced neurodegeneration in NE-4C cells.







Similar content being viewed by others
References
Calne DB, Chu NS, Huang CC, Lu CS, Olanow WMD (1994) Manganism and idiopathic parkinsonism: similarities and differences. Neurology 44:1583–1586
Gallez B, Baudalet C, Adline J, Geurts M, Delzenne N (1997) Accumulation of manganese in the brain of mice after intravenous injection of manganese-based contrast agents. Chem Res Toxicol 10:360–363
Lenter C (1981) Composition of the body. In: Geigy scientific tablesI Ed. Ciba -Geigy Limited, Basle pp 22
Golub MS, Han B, Keen CL, Gershwin ME (1992) Effects of dietary aluminium excess and manganese deficiency on neurobehavioral end points in adult mice. Toxicol Appl Pharmacol 112:154–160
Gianutsos GS, Saymeth R, Wu ML, Michel RG (1985) Brain manganese accumulation following systemic administration of different forms. Arch Toxicol 57:272–275
Donaldson JMG, La Bella FS (1982) Manganese neurotoxicity: a model for free radical mediated neurodegeneration. Can J Physiol Pharmacol 60:1398–1405
Chua ACG, Morgan EH (1996) Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol Trace Elem Res 55:39–55
Pennington JAT, Young BE (1991) Total diet study nutritional elements. J Am Diet Assoc 91:179–183
Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W (1995) Manganese-induced reactive oxygen species: comparison between Mn + 2 and Mn + 3. Neurodegeneration 4:329–334
Collipp PJ, Chen SY, Maitinsky S (1983) Manganese in infant formulas and learning disability. Ann Nutr Metab 27:488–494
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Lorincz C, Karpati E, Szporny L, Szasz K, Kisfaludy L (1973) Alkaloid esters. Ger. Offen. 2 253 750. Chem Abstr 79:42726
Araki T, Kato H, Kogure K (1991) Comparative protective effectsof vinconate, baclofen, and pentobarbital against neuronal damage following repeated brief cerebral ischemia in the gerbil brain. Res Exp Med (Berl) 191:371–378
Erdö SL, Cai NS, Wolff JR, Kiss B (1990) Vinpocetine protects against excitotoxic cell death in primary cultures of rat cerebral cortex. Eur J Pharmacol 187(3):551–553
Dogrukol Ak D, Banks WA, Tuncel N, et al. (2003) Passage of vasoactive intestinal peptide across the blood-brain barrier. Peptides 24(3):437–444
Tamm C, Sabri F, Ceccatelli S (2008) Mitochondrial-mediated apoptosis in neural stem cells exposed to manganese. Toxicol Sci 101(2):310–320
Korkmaz OT, Tunçel N, Tunçel M, Oncü EM, Sahintürk V, Celik M (2010) Vasoactive intestinal peptide (VIP) treatment of parkinsonian rats increases thalamic gamma-aminobutyric acid (GABA) levels and alters the release of nerve growth factor (NGF) by mast cells. J Mol Neurosci 41(2):278–287
Offen D, Sherki Y, Melamed E, Fridkin M, Brenneman DE, Gozes I (2000) Vasoactive intestinal peptide (VIP) prevents neurotoxicity in neuronal cultures: relevance to neuroprotection in Parkinson’s disease. Brain Res 854(1–2):257–262
Milatovic D, Gupta R, Yu Y, Zaja-Milatovic S, Aschner M (2011) Protective effects of antioxidants and anti-inflammatory agents against manganese-induced oxidative damage and neuronal injury. Toxicol Appl Pharmacol 256(3):219–226
Dobson AW, Erikson KM, Aschner M (2004) Manganese neurotoxicity. Ann N Y Acad Sci 1012:115–128
Levy BS, Nassetta WJ (2003) Neurologic effects of manganese in humans: a review. Int J Occup Environ Health 9:153–163
Sagvolden T, Johansen EB, Aase H, Russell VA (2005) A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci 28:397–419
Overmeyer S, Bullmore ET, Suckling J, Simmons A, Williams SC, Santosh PJ, Taylor E (2001) Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychol Med 31:1425–1435
Vettori MV, Gatti R (1999) An in vitro model for the assessment of manganese neurotoxicity. Toxicol in Vitro 13(6):931–938
Marreilha dos Santos AP, Santos D, Au C, Milatovic D, Aschner M, Batoréu MC (2008) Antioxidants prevent the cytotoxicity of manganese in RBE4 cells. Brain Res 1236:200–205
Strederick DL et al. (2004) Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology 25:543–553
Reaney SH, Smith DR (2005) Manganese oxidation state mediates toxicity in PC12 cells. Toxicol Appl Pharmacol 205:271–281
Krysko DV, Roels F, Leybaert L, D’Herde K (2001) Mitochondrial transmembrane potential changes support the concept of mitochondrial heterogeneity during apoptosis. J Histochem Cytochem 49(10):1277–1284
Lee ES, Yin Z, Milatovic D, Jiang H, Aschner M (2009) Estrogen and tamoxifen protect against Mn-induced toxicity in rat cortical primary cultures of neurons and astrocytes. Toxicol Sci 110:156–167
Ashner M, Ashner JL (1991) Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Nerosci Behav Rev 15:333–340
Pennington JAT, Young BE, Wilson DB (1989) Nutritional elements in U.S diets: results for the total diet study. J Am Diet Assoc 89:659–664
Santos AP, Lucas RL, Andrade V, Mateus ML, Milatovic D, Aschner M, Batoreu MC (2012) Protective effects of ebselen (Ebs) and para-aminosalicylic acid (PAS) against manganese (Mn)-induced neurotoxicity. Toxicol Appl Pharmacol 258(3):394–402
Hernández RB, Farina M, Espósito BP, Souza-Pinto NC, Barbosa F Jr, Suñol C (2011) Mechanisms of manganese-induced neurotoxicity in primary neuronal cultures: the role of manganese speciation and cell type. Toxicol Sci 124(2):414–423
Lobner D (2000) Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis. J Neurosci Methods 96(2):147–152
Shoge K, Hiromu K (1998) Protective effects of vasoactive intestinal peptide against delayed glutamate neurotoxicity in cultured retina. Brain Res 809:127–136
Solanki P et al. (2011) Preventive effect of piracetam and vinpocetine on hypoxia-reoxygenation induced injury in primary hippocampal culture. Food Chem Toxicol 49:917–922
Pendergrass W, Wolf N, Poot M (2004) Efficacy of MitoTracker Green™ and CMX Rosamine to measure changes in mitochondrial membrane in living cells and tissues. Cytometry A 61(2):162–169
Alaimo A, Gorojod RM, Miglietta EA, Villarreal A, Ramos AJ, Kotler ML (2013) Manganese induces mitochondrial dynamics impairment and apoptotic cell death: a study in human Gli36 cells. Neurosci Lett 554:76–81
Gonzalez LE, Juknat AA, Venosa AJ, Verrengia N, Kotler ML (2008) Manganese activates the mitochondrial apoptotic pathway in rat astrocytes by modulating the expression of proteins of the bcl-2 family. Neurochem Int 53(6–8):408–415
Acknowledgments
This study was supported by the Ege University Scientific Research Foundation (Project No: 13/TIP/003). The authors also acknowledge the Pharmaceutical Sciences Research Centre (FABAL) of Ege University, Faculty of Pharmacy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s12011-016-0775-3.
Rights and permissions
About this article
Cite this article
Bora, S., Erdogan, M.A., Armagan, G. et al. Vinpocetine and Vasoactive Intestinal Peptide Attenuate Manganese-Induced Toxicity in NE-4C Cells. Biol Trace Elem Res 174, 410–418 (2016). https://doi.org/10.1007/s12011-016-0742-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0742-z