Abstract
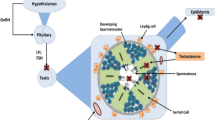
This study investigated the effect of copper sulfate (CuSO4) in the rat spermatogenesis. Forty male rats, weighing 70–80 g, were randomly divided into four groups: control group (CG, 0 mg/kg BW), low-dose group (LG, 100 mg/kg BW), mid-dose group (MG, 200 mg/kg BW), and high-dose group (HG, 400 mg/kg BW). Rats were administered CuSO4 by gavage for 30 days. A variety of measurements were taken including the testis coefficients, the sperm count, the abnormal malformation rate, testosterone (T), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) concentrations in the serum. In addition, glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) activities, and malondialdehyde (MDA) concentration in the testis were determined. The results showed that in the CuSO4-treated groups, the testis coefficients, sperm count, T, LH, and FSH concentrations, and GSH-Px and SOD activities decreased, while the abnormal malformation rate and MDA concentration increased, compared with the CG. It indicates that CuSO4 exposure impairs the sperm quality and inhibits secretion of sex hormone and gonadotropin, and testis anti-oxidative function, suppressing the rat spermatogenesis.




Similar content being viewed by others
References
Elgerwi A, Bires J, Levkut M (1999) Industrial copper intoxication in sheep: clinical and pathological findings. Acta. Vet. Brno 68:197–202
Wong WY, Flik G, Groenen PMW, Swinkels DW, Thomas CMG, Copius-Peereboom JHJ, Merkus HMWM, SteegersTheunissen RPM (2001) The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol 15:131–136
Skandhan KP (1992) Review on copper in male reproduction and contraception. Rev Fr Gynecol Obstet 87:594–598
Boubsil S, Abdennour C, Tegurin M (2011) Assessment of some blood biomarkers of workmen from a copper wire factory. Annals Biol Res 2:164–169
Bidewell C, Livesey C (2002) Copper poisoning: an emerging disease in dairy cattle. State Vet J 12:16–19
Bedwal RS, Bahuguna A (1994) Zinc, copper and selenium in reproduction. Experientia 50(7):626–640
Rebrelo L, Guadarrama A, Lopez T, Zegers HF (1996) Effect of Cu ion on the motility, viability, acrosome reaction and fertilizing capacity of human spermatozoa in vitro. Reprod Fertil 8:871–874
Negri AP, Heyward AJ (2001) Inhibition of coral fertilisation and larval metamorphosis by tributyltin and copper. Mar Environ Res 51:17–27
Victor S, Richmond RH (2005) Effect of copper on fertilization success in the reef coral Acropora surculosa. Mar Pollut Bull 50:1448–1451
Hédouin L, Gates RD (2013) Assessing fertilization success of the coral Montipora capitata under copper exposure: does the night of spawning matter? Mar Pollut Bull 66:221–224
Knazicka Z, Tvrda E, Bardos L, Lukac N (2012) Dose- and time-dependent effect of copper ions on the viability of bull spermatozoa in different media. J Environ Sci Health A Tox Hazard Subst Environ Eng. 47:1294–1300
Gharred T, Ezzine IK, Naija A, Bouali RR, Jebali J (2015) Assessment of toxic interactions between deltamethrin and copper on the fertility and developmental events in the Mediterranean sea urchin Paracentrotus lividus. Environ Monit Assess 187:193
Aydemir B, Kiziler AR, Onaran I, Alici B, Ozkara H, Akyolcu MC (2006) Impact of Cu and Fe concentration on oxidative damage in male infertility. Biol Trace Elem Res 112:193–203
Salsabili N, Mehrsai AR, Jalaie S (2009) Concentration of blood and seminal plasma elements and their relationships with semen parameters in men with spinal cord injury. Andrologia 41:24–28
Gaur M, Pruthi V, Prasad R, Pereira BM (2000) Inductively coupled plasma emission spectroscopic and flame photometric analysis of goat epididymal fluid. Asian J Androl 2:288–292
Babaei H, Abshenas J (2013) Zinc therapy improves adverse effects of long term administration of copper on epididymal sperm quality of rats. Iran J Reprod Med (11):577–582
Sakhaee E, Emadi L, Abshenas J, Kheirandish R, Azari O, Amiri E (2012) Evaluation of epididymal sperm quality following experimentally induced copper poisoning in male rats. Andrologia 44:110–116
Miska-Schramm A, Kruczek M, Kapusta J (2014) Effect of copper exposure on reproductive ability in the bank vole (Myodes glareolus). Ecotoxicology 23:1546–1554
Orgebin-Crist MC, Tichenor PL (1973) Effect of testosterone on sperm maturation in vitro. Nature 245(5424):328–329
Ahmed SDH, Ahsan S, Burney SIAB (2013) Male fertility: influence of testosterone, luteinizing hormone, and follicle-stimulating hormone on seminal free L-carnitine. Hum Androl 3:76–80
Chang CS, Choi JB, Kim HJ, Park SB (2011) Correlation between serum testosterone level and concentrations of copper and zinc in hair tissue. Biol Trace Elem Res 144:264–271
Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163
Aitken RJ, Baker MA (2006) Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol 250(1–2):66–69
Zhang S, Chong H, Chang L, Wang SH (2014) The effects of Stellera chamaejasme on superoxide dismutase, glutathione peroxidase and malondialdehyde in the tissues of white mice. Heilongjiang Animal Science and Veterinary Medicine 5:105–107
Tvrda E, Peer R, Sikka SC, Agarwal A (2015) Iron and copper in male reproduction: a double-edged sword. J Assist Reprod Genet 3:3–16
Roy D, Dey S, Majumder GC, Bhattacharyya D (2014) Copper: a biphasic regulator of caprine sperm forward progression. Syst Biol Reprod Med 60:52–57
Zhu YZ, Sun H, Fu Y, Wang J, Song M, Li M, Li YF, Miao LG (2014) Effects of sub-chronic aluminum chloride on spermatogenesis and testicular enzymatic activity in male rats. Life Sci 102:36–40
Kong YQ, Chen LQ, Li EC, Du ZY, Ding ZL (2014) Growth and antioxidant activity of juvenile oriental river prawn Macrobrachium nipponense, fed diets containing different copper levels under nitrite exposure. Global Adv Res J Agricul Sci 3:119–127
Babaei H, Kheirandishb R, Ebrahimic L (2012) The effects of copper toxicity on histopathological and morphometrical changes of the rat testes. Asian Pac J Trop Med 2:S1615–S1619
Kheirandish R, Askari N, Babaei H (2014) Zinc therapy improves deleterious effects of chronic copper administration on mice testes: histopathological evaluation. Andrologia 46:80–85
Slivkova J, Popelkova M, Massanyi P, Toporcerova S, Stawarz R, Formicki G, Lukac N, Putała A, Guzik M (2009) Concentration of trace elements in human semen and relation to spermatozoa quality. J Environ Sci Health A Tox Hazard Subst Environ Eng 44:370–375
Holland MK, White IG (1982) Heavy metals and human spermatozoa: II. The effect of seminal plasma on the toxicity of copper metal for spermatozoa. Int J Fertil 27:95–99
Stohs ST, Bagchi D (1995) Oxidative mechanisms in the toxicity of metals. Free Radic Biol Med 18:321–326
Dupont CL, Grass G, Rensing C (2011) Copper toxicity and the origin of bacterial resistance—new insights and applications. Metallomics 3:1109–1118
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. In: Kenneth BS (ed) Functional metabolism: regulation and adaptation. John Wiley and Sons, Inc, pp. 319–368
Muralidhara BS (2007) Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol 23:578–587
Aitken RJ, Backer HWG, Irvine DS (1995) On the nature of semen quality and infertility. Hum Reprod 10:248–249
Cummins JM, Jequier AM, Kan R (1994) Molecular biology of human male infertility: links with aging, mitochondrial genetics and oxidative stress? Mol Reprod Dev 37:345
Jenkins KJ (1989) Effect of copper loading of preruminant calves on intracellular distribution of hepatic copper, zinc, iron, and molybdenum. J Dairy Sci 72:2346–2350
Chillappagari S, Seubert A, Trip H, Kuipers OP, Marahiel MA, Miethke M (2010) Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in bacillus subtilis. J Bacteriol 192:2512–2524
Acknowledgments
This study was supported by a grant from the Ph.D. Programs Foundation of Ministry of Education of China (20132325110001), the Natural Science Foundation of Heilongjiang Province of China (C201425), and the National Natural Science Foundation Project (31372496).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Liu, J.Y., Yang, X., Sun, X.D. et al. Suppressive Effects of Copper Sulfate Accumulation on the Spermatogenesis of Rats. Biol Trace Elem Res 174, 356–361 (2016). https://doi.org/10.1007/s12011-016-0710-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0710-7