Abstract
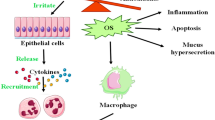
Arsenic is present in water, soil, and air in organic as well as in inorganic forms. However, inorganic arsenic is more toxic than organic and can cause many diseases including cancers in humans. Its genotoxic effect is considered as one of its carcinogenic actions. Arsenic can cause DNA strand breaks, deletion mutations, micronuclei formation, DNA-protein cross-linking, sister chromatid exchange, and DNA repair inhibition. Evidences indicate that arsenic causes DNA damage by generation of reactive free radicals. Nutritional supplementation of antioxidants has been proven highly beneficial against arsenic genotoxicity in experimental animals. Recent studies suggest that antioxidants protect mainly by reducing excess free radicals via restoring the activities of cellular enzymatic as well as non-enzymatic antioxidants and decreasing the oxidation processes such as lipid peroxidation and protein oxidation. The purpose of this review is to summarize the recent literature on arsenic-induced genotoxicity and its mitigation by naturally derived antioxidants in various biological systems.
Similar content being viewed by others
Abbreviations
- NRC:
-
National Research Council
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GSH:
-
Glutathione
- ALAD:
-
δ-Aminolevulinic acid dehydratase
- DMSA:
-
Meso-2,3-dimercaptosuccinic acid
- δ-ALA:
-
δ-Aminolevulinic acid
- GPx:
-
Glutathione peroxidase
- SAM:
-
S-Adenosyl methionine
- MDA:
-
Malondialdehyde
- XO:
-
Xanthine oxidase
References
Waalkes MP, Fox DA, States JC, Patierno SR, McCabe MJ Jr (2000) Metals and disorders of cell accumulation: modulation of apoptosis and cell proliferation. Toxicol Sci 56:255–261
Knowles FC, Benson AA (1984) The enzyme inhibitory form of inorganic arsenic. Z Gesamten Hyg 30:625–626
Bettley FR, O’Shea JA (1975) The absorption of arsenic and its relation to carcinoma. Br J Dermatol 92:563–568
Landolph JR (1994) Molecular mechanisms of transformation of C3H/10T1/2 C1 8 mouse embryo cells and diploid human fibroblasts by carcinogenic metal compounds. Environ Health Perspect 102:119–125
Chen CJ, Wang CJ (1990) Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res 50:5470–5474
Matschullat J (2000) Arsenic in the geosphere—a review. Sci Total Environ 249:297–312
NRC (1999) Arsenic in the drinking water. National Research Council, National Academy Press, Washington, D.C
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Mitra AK, Bose BK, Kabir H, Das BK, Hussain M (2002) Arsenic-related health problems among hospital patients in southern Bangladesh. J Health Popul Nutr 20:198–204
Pandey PK, Yadav S, Nair S, Bhui A (2002) Arsenic contamination of the environment: a new perspective from central-east India. Environ Int 28:235–245
Chowdhury UK, Biswas BK, Chowdhury TR et al (2000) Ground water arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect 108:393–397
Nordstrom DK (2002) Worldwide occurrences of arsenic in ground water. Science 296:2143–2145
United States Environmental Protection Agency (2001) National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Fed Regist 66:6976–7066
Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE (1996) Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect 104:620–628
Tam GK, Charbonneau SM, Bryce F, Pomroy C, Sandi E (1979) Metabolism of inorganic arsenic (74As) in humans following oral ingestion. Toxicol Appl Pharmacol 50:319–322
Foa V, Colombi A, Maroni M, Buratti M, Calzaferri G (1984) The speciation of the chemical forms of arsenic in the biological monitoring of exposure to inorganic arsenic. Sci Total Environ 34:241–259
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
Mandal BK, Ogra Y, Suzuki KT (2001) Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic-affected areas in West Bengal, India. Chem Res Toxicol 14:371–378
Vahter M, Concha G (2001) Role of metabolism in arsenic toxicity. Pharmacol Toxicol 89:1–5
Shi H, Shi X, Liu KJ (2004) Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem 255:67–78
Valko M, Morris H, Cronin MT (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Martinez VD, Vucic EA, Adonis M, Gil L, Lam WL (2011) Arsenic biotransformation as a cancer promoting factor by inducing DNA damage and disruption of repair mechanisms. Mol Biol Int 2011, 718974. doi:10.4061/2011/718974
Gonsebatt ME, Vega L, Salazar AM, Montero R, Guzman P, Blas J et al (1997) Cytogenetic effects in human exposure to arsenic. Mutat Res 386(Suppl 3):219–228
Biggs ML, Kalman DA, Moore LE, Hopenhayn-Rich C, Smith MT, Smith AH (1997) Relationship of urinary arsenic to intake estimates and a biomarker of effect, bladder cell micronuclei. Mutat Res 386:185–195
Tinwell H, Stephens SC, Ashby J (1991) Arsenite as the probable active species in the human carcinogenicity of arsenic: mouse micronucleus assays on Na and K arsenite, orpiment, and Fowler’s solution. Environ Health Perspect 95:205–210
Barrett JC, Lamb PW, Wang TC, Lee TC (1989) Mechanisms of arsenic-induced cell transformation. Biol Trace Elem Res 21:421–429
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2004) Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum 84:1–477
IARC (1980) Arsenic and arsenic compounds. Some metals and metallic compounds. IARC Monogr Eval Carcinog Risks Hum 23:39–141
IARC (1987) Arsenic. Overall evaluation of carcinogenicity: an updating of IARC monographs Volumes 1–42. International Agency for Research on Cancer, Lyon, pp 100–106, Suppl 7
Leonard A, Lauwerys RR (1980) Carcinogenicity, teratogenicity, and mutagenicity of arsenic. Mutat Res 75:49–62
Tsai SM, Wang TS, Ko YC (1999) Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Environ Health 54(Suppl 3):186–193
Smith AH, Arroyo AP, Mazumder DNG, Kosnett MJ, Hernandez AL, Beeris M et al (2000) Arsenic-induced skin lesions among Atacameno people in Northern Chile despite good nutrition and centuries of exposure. Environ Health Perspect 108(Suppl 7):617–620
Col M, Col C, Soran A, Sayli BS, Ozturk S (1999) Arsenic related Bowen’s disease, palmar keratosis, and skin cancer. Environ Health Perspect 107(Suppl 8):687–689
Ahmad S, Kitchin KT, Cullen WR (2000) Arsenic species that causes release of iron from ferritin and generation of activated oxygen. Arch Biochem Biophys 382:195–202
Hei TK, Liu SX, Waldren C (1998) Mutagenicity of arsenic in mammalian cells: role of reactive oxygen species. Proc Natl Acad Sci U S A 95:8103–8107
Li D, Morimoto K, Takeshita T, Lu Y (2001) Arsenic induces DNA damage via reactive oxygen species in human cells. Environ Health Prev Med 6:27–32
Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B, Goyer R, Menzer R, Rossman T, Thompson C, Waalkes M (1999) Arsenic: health effects, mechanisms of actions, and research issues. Environ Health Perspect 107:593–597
Lynn S, Gurr JR, Lai HT, Jan KY (2000) NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Circ Res 86:514–519
Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang IH, Chen CJ, Lee TC (2001) Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in a human population of northeastern Taiwan. Environ Health Perspect 1091:1011–1017
Kitchin KT, Wallace K (2008) Evidence against the nuclear in situ binding of arsenicals—oxidative stress theory of arsenic carcinogenesis. Toxicol Appl Pharmacol 232:252–257
Bau DT, Wang TS, Chung CH, Wang AS, Jan KY (2002) Oxidative DNA adducts and DNA-protein cross-links are the major DNA lesions induced by arsenite. Environ Health Perspect 110:753–756
Hwang ES, Kim GH (2007) Biomarkers for oxidative stress status of DNA, lipids, and proteins in vitro and in vivo cancer research. Toxicology 229:1–10
Li JH, Rossman TG (1989) Mechanism of comutagenesis of sodium arsenite with n-methyl-n-nitrosourea. Biol Trace Elem Res 21:373–381
Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP (1997) Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci U S A 94:10907–10912
Dizik M, Christman JK, Wainfan E (1991) Alterations in expression and methylation of specific gene in livers of rats fed a cancer promoting methyl-deficient diet. Carcinogenesis 12(Suppl 7):1307–1312
Christman JK, Sheikhnejad G, Dizik M, Abileah S, Wainfan E (1993) Reversibility of changes in nucleic acid methylation and gene expression induced in rat liver by severe dietary deficiency. Carcinogenesis 14(Suppl 4):551–557
Hsieh LL, Wainfan E, Hoshina S, Dizik M, Weinstein IB (1989) Altered expression of retrovirus-like sequences and cellular oncogenes in mice fed methyl-deficient diets. Cancer Res 49:3795–3799
Mass MJ, Wang L (1997) Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for mechanism of carcinogenesis. Mutat Res 386:263–277
Flora SJS, Bhadauria S, Kannan GM, Singh N (2007) Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J Environ Biol 28:333–347
Kannan GM, Flora SJS (2004) Chronic arsenic poisoning in the rats: treatment with combined administration of succimers and an antioxidants. Ecotoxicol Environ Saf 58:37–43
Mishra D, Flora SJS (2008) Quercetin administration during chelation therapy protects arsenic-induced oxidative stress in mice. Biol Trace Elem Res 122(2):137–147
Milton Prabu S, Muthumani M (2012) Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep 39:11201–11216
Mershiba SD, Dassprakash MV, Saraswathy SD (2013) Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep 40:3681–3691
Kaur H, Mishra D, Bhatnagar P, Kaushik P, Flora SJS (2009) Co-administration of α-lipoic acid and vitamin C protects liver and brain oxidative stress in mice exposed to arsenic contaminated water. Water Qual Expo Health 1:135–144
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Pant H, Rao MV (2010) In vitro melatonin supplementation against genetic toxicity by arsenic and fluoride. Adv Biores 1(2):17–24
Singh MK, Yadav SS, Gupta V, Khattri S (2013) Immunomodulatory role of Emblica officinalis in arsenic induced oxidative damage and apoptosis in thymocytes of mice. BMC Complement Alternat Med 13:193
Balakumar BS, Ramanathan K, Kumaresan S, Suresh R (2010) DNA damage by sodium arsenite in experimental rats: ameliorative effects of antioxidant vitamins C and E. Indian J Sci Technol 3(3):322–327
Muthumani M (2013) Tetrahydrocurcumin potentially attenuates arsenic induced oxidative hepatic dysfunction in rats. J Clin Toxicol 3, 168. doi:10.4172/2161-0495.1000168
Winship KA (1984) Toxicity of inorganic arsenic salts. Adverse Drug React Acute Poisoning Rev 3:129–160
Mandal BK, Chowdhury TR, Samanta G et al (1996) Arsenic in groundwater in seven districts of west Bengal, India: the biggest arsenic calamity in the world. Curr Sci 70:976–986
Rahman M, Vahter M, Wahed MA et al (2006) Prevalence of arsenic exposure and skin lesions. A population based survey in Matlab, Bangladesh. J Epidemiol Community Health 60:242–248
Hunter FT, Kip AF, Irvine W (1942) Radioactive tracer studies on arsenic injected as potassium arsenite. J Pharmacol Exp Ther 76:207
NIOSH (1984) National Occupational Exposure Survey (1980–1983). Department of Health and Human Services. National Institute for Occupational Safety and Health, Cincinnati, OH
Lansdown AB (1995) Physiological and toxicological changes in the skin resulting from the action and interaction of metal ions. Crit Rev Toxicol 25:397–462
Luchtrath H (1983) The consequences of chronic arsenic poisoning among Moselle wine growers. Pathoanatomical investigations of post-mortem examinations performed between 1960 and 1977. J Cancer Res Clin Oncol 105:173–182
Philipp R (1985) Arsenic exposure: health effects and the risk of cancer. Rev Environ Health 5:27–57
Yamauchi H, Yamamura Y (1983) Concentration and chemical species of arsenic in human tissue. Bull Environ Contam Toxicol 31:267–270
Lindgren A, Vahter M, Dencker L (1982) Autoradiographic studies on the distribution of arsenic in mice and hamsters administered 74As-arsenite or -arsenate. Acta Pharmacol Toxicol (Copenh) 51:253–265
Longnecker MP, Daniels JL (2001) Environmental contaminants as etiologic factors for diabetes. Environ Health Perspect 109(Suppl 6):871–876
Tseng CH, Tseng CP, Chiou HY, Hsueh YM, Chong CK, Chen CJ (2002) Epidemiologic evidence of diabetogenic effect of arsenic. Toxicol Lett 133:69–76
Wang A, Holladay SD, Wolf DC, Ahmed SA, Robertson JL (2006) Reproductive and developmental toxicity of arsenic in rodents: a review. Int J Toxicol 25:319–331
Sarkar M, Chaudhuri GR, Chattopadhyay A, Biswas NM (2003) Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J Androl 5:27–31
Aposhian HV (1996) Arsenic toxicology: does methylation of arsenic species have an evolutionary significance? Met Ions Biol Med 4:399–401
Kitchin KT (2001) Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol 172:249–261
Basu A, Ghosh P, Das JK, Banerjee A, Ray K, Giri AK (2004) Micronuclei as biomarkers of carcinogen exposure in populations exposed to arsenic through drinking water in west Bengal, India: a comparative study in three cell types. Cancer Epidemiol Biomarkers Prev 13:820–827
Wegner R, Radon K, Heinrich-Ramm R, Seemann B, Riess A, Koops F, Poschadel B, Szadkowski D (2004) Biomonitoring results and cytogenetic markers among harbour workers with potential exposure to river silt aerosols. Occup Environ Med 61:247–253
Chen CJ, Hsu LI, Wang CH, Shih WL et al (2005) Biomarkers of exposure, effect, and susceptibility of arsenic-induced health hazards in Taiwan. Toxicol Appl Pharmacol 206:198–206
Martinez V, Creus A, Venegas W, Arroyo A, Beck JP, Gebel TW, Surralles J, Marcos R (2005) Micronuclei assessment in buccal cells of people environmentally exposed to arsenic in northern Chile. Toxicol Lett 155:319–327
Ramirez T, Garcia-Montalvo V, Wise C, Cea-Olivares R, Poirier LA, Herrera LA (2003) S-adenosyl-L-methionine is able to reverse micronucleus formation induced by sodium arsenite and other cytoskeleton disrupting agents in cultured human cells. Mutat Res 528:61–74
Dopp E, Hartmann LM, Florea AM, Von Recklinghausen U, Pieper R, Shokouhi B, Rettenmeier AW, Hirner AV, Obe G (2004) Uptake of inorganic and organic derivatives of arsenic associated with induced cytotoxic and genotoxic effects in Chinese hamster ovary (CHO) cells. Toxicol Appl Pharmacol 201:156–165
Colognato R, Coppede F, Ponti J, Sabbioni E, Migliore L (2007) Genotoxicity induced by arsenic compounds in peripheral human lymphocytes analysed by cytokinesis-block micronucleus assay. Mutagenesis 22:255–261
Mahata J, Ghosh P, Sarkar JN, Ray K, Natarajan AT, Giri AK (2004) Effect of sodium arsenite on peripheral lymphocytes in vitro: individual susceptibility among a population exposed to arsenic through the drinking water. Mutagenesis 19(3):223–229
Yih LH, Lee TC (2000) Arsenite induces p53 accumulation through an ATM-dependent pathway in human fibroblasts. Cancer Res 60:6346–6352
Vuyyuri SB, Ishaq M, Kuppala D, Grover P, Ahuja YR (2006) Evaluation of micronucleus frequencies and DNA damage in glass workers exposed to arsenic. Environ Mol Mutagen 47:562–570
Ding W, Hudson LG, Liu KJ (2005) Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol Cell Biochem 279:105–112
Ahmed K, Akhand AA, Hasan M, Islam M, Hasan A (2008) Toxicity of arsenic (sodium arsenite) to fresh water spotted snakehead Channa punctatus (Bloch) on cellular death and DNA content. Am-Euras J Agric Environ Sci 4(1):18–22
Partridge MA, Huang SXL, Hernandez-Rosa E, Davidson MM, Hei TK (2007) Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res 67:5239–5247
Huang SC, Lee TC (1998) Arsenite inhibits mitotic division and perturbs spindle dynamics in HeLa S3 cells. Carcinogenesis 19:889–896
Nakamuro K, Sayato T (1981) Comparative studies of chromosomal aberration induced by trivalent and pentavalent arsenic. Mutat Res 88:73–80
Nordenson I, Sweins A, Beckman L (1981) Chromosome aberrations in cultured human lymphocytes exposed to trivalent and pentavalent arsenic. Scand J Work Environ Health 7:277–281
Schwerdtle T, Walter I, Mackiw I, Hartwig A (2003) Induction of oxidative DNA damage by arsenite and its trivalent and pentavalent methylated metabolites in cultured human cells and isolated DNA. Carcinogenesis 24(5):967–974
Sordo M, Herrera LA, Ostrosky-Wegman P, Rojas E (2001) Cytotoxic and genotoxic effects of As, MMA, and DMA on leukocytes and stimulated human lymphocytes. Teratog Carcinog Mutagen 21:249–260
Wang TS, Hsu TY, Chung CH, Wang AS, Bau DT, Jan KY (2001) Arsenite induces oxidative DNA adducts and DNA-protein cross-links in mammalian cells. Free Radic Biol Med 31:321–330
Dong JT, Luo XM (1993) Arsenic-induced DNA-strand breaks associated with DNA-protein crosslinks in human fetal lung fibroblasts. Mutat Res 302:97–102
Ramirez P, Del Razo LM, Gutierrez-Ruiz MC, Gonsebatt ME (2000) Arsenite induces DNA-protein crosslinks and cytokeratin expression in the WRL-68 human hepatic cell line. Carcinogenesis 21:701–706
Pierce BL, Kibriya MG, Tong L, Jasmine F et al (2012) Genome-wide association study identifies chromosome 10q24.32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS Genet 8, e1002522. doi:10.1371/journal.pgen.1002522
Helleday T, Nilsson R, Jenssen D (2000) Arsenic[III] and heavy metal ions induce intrachromosomal homologous recombination in the hprt gene V79 Chinese hamster cells. Environ Mol Mutagen 35:114–122
Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, Kligerman AD (2001) Methylated trivalent arsenic species are genotoxic. Chem Res Toxicol 14:355–361
Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H (2000) Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol 163:203–207
Petrick JS, Jagadish B, Mash EA, Aposhian HV (2001) Monomethylarsonous acid (MMAIII) and arsenite: LD50 in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol 14:651–656
Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ (2000) Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol 74:289–299
Andrew AS, Karagas MR, Hamilton JW (2003) Decreased DNA repair gene expression among individuals exposed to arsenic in United States drinking water. Int J Cancer 104:263–268
Li JH, Rossman TG (1989) Inhibition of DNA ligase activity by arsenite: a possible mechanism of its comutagenesis. Mol Toxicol 2:1–9
Bencko V, Wagner V, Wagnerova M, Botora J (1988) Immunological profiles in workers of a power plant burning coal rich in arsenic content. J Hyg Epidemiol Microbiol Immunol 32:137–147
Nordenson I, Salmonsson S, Brun E, Bechman G (1978) Occupational and environmental risks in and around a smelter in northern Sweden. II. Chromosomal aberrations in workers exposed to arsenic. Hereditas 88:47–50
Rossman TG, Meyn MS, Troll W (1977) Effects of arsenic on DNA repair in Escherichia coli. Environ Health Perspect 19:229–233
Sinha D, Roy M (2011) Antagonistic role of tea against sodium arsenite-induced oxidative DNA damage and inhibition of DNA repair in Swiss albino mice. J Environ Pathol Toxicol Oncol 30:311–322
Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzalez MB, Garcia-Montalvo EA, Calderon-Aranda ES, Del Razo LM (2005) Urinary trivalent methylated arsenic species in a population chronically exposed to inorganic arsenic. Environ Health Perspect 113:250–254
Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, Styblo M, Le XC, Creed JT, Maeda N, Hughes MF, Thomas DJ (2009) Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem Res Toxicol 22:1713–1720
Hall AH (2002) Chronic arsenic poisoning. Toxicol Lett 128:69–72
Naranmandura H, Suzuki N, Suzuki KT (2006) Trivalent arsenicals are bound to proteins during reductive methylation. Chem Res Toxicol 19:1010–1018. doi:10.1021/tx060053f
Suzuki KT, Mandal BK, Ogra Y (2002) Speciation of arsenic in body fluids. Talanta 58:111–119. doi:10.1016/ S0039-9140(02)00260-6
Garcia-Shavez E, Jimenez I, Segura B, Razo LMD (2006) Lipid peroxidative damage and distribution of inorganic and its metabolite in the rat nervous system after arsenite exposure: influence of alpha tocopherol. Neurotoxicology 27:1024–1031
Kessel M, Liu SX, Xu A, Santella R, Hei TK (2002) Arsenic induces oxidative DNA damage in mammalian cells. Mol Cell Biochem 234(235):301–308
Flora SJ (2011) Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51:257–281
Herman JG, Merlo A, Mao L, Lapidus RG, Issa JPJ, Davidson NE (1995) Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res 55:4525–30
Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24(5A):2783–2840
Chen C, Jiang X, Hu Y, Zhang Z (2013) The protective role of resveratrol in the sodium arsenite-induced oxidative damage via modulation of intracellular GSH homeostasis. Biol Trace Elem Res 155:119–131
Singh N, Kumari D (2013) Amelioration of genotoxicity by papaya extract induced by arsenic contaminated drinking water. Biogeosciences 8(2):623–626
Manna P, Sinha M, Sil PC (2007) Protection of arsenic-induced hepatic disorder by arjunolic acid. Basic Clin Pharmacol Toxicol 101:333–338
Acharyya N, Ali SS, Deb B, Chattopadhyay S, Maiti S (2014) Green tea (Camellia sinensis) alleviates arsenic-induced damages to DNA and intestinal tissues in rat and in situ intestinal loop by reinforcing antioxidant system. Environ Toxicol. doi:10.1002/tox
Zhu QY, Chen ZY (1999) Isolation and analysis of green tea polyphenols by HPLC. Anal Lab 18:70–72
Bhattacharya S, Haldar PK (2012) Ameliorative effect Trichosanthes dioica root against arsenic-induced brain toxicity in albino rats. Toxicol Environ Chem 94(4):769–778
Bhattacharya S, Haldar PK (2012) Trichosanthes dioica fruit ameliorates experimentally induced arsenic toxicity in male albino rats through the alleviation of oxidative stress. Biol Trace Elem Res 148:232–241
Chopra RN, Nayar SL, Chopra IC (2002) Glossary of Indian medicinal plants, 1st edn. CSIR, New Delhi, pp 340–1
Ghaisas MM, Tanwar MB, Ninave PB, Navghare VV, Deshpande AD (2008) Hepatoprotective activity of aqueous and ethanolic extract of Trichosanthes dioica Roxb. in ferrous sulphate induced liver injury. Pharmacologyonline 3:127–35
Maiti S, Chattopadhyay S, Acharyya N, Deb B, Hati AK (2014) Emblica officinalis (amla) ameliorates arsenic induced liver damage via DNA protection by antioxidants systems. Mol Cell Toxicol 10:75–82
Khan KH (2009) Roles of Emblica officinalis in medicine—a review. Bot Res Int 2(4):218–28
Habib-ur-Rehman YKA, Choudhary MA, Khaliq N, Atta-ur-Rahman CMI, Malik S (2007) Studies on the chemical constituents of Phyllanthus emblica. Nat Prod Res 21:775–81
Krishnaveni M, Mirunalini S (2010) Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol 21:93–105
Chattopadhyay S, Maiti S, Maji G, Deb B, Pan B, Ghosh D (2011) Protective role of Moringa oleifera (Sajina) seed on arsenic-induced hepatocellular degeneration in female albino rats. Biol Trace Elem Res 142:200–212
Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, Upadhyay G, Singh HB (2009) Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol 47:1109–1116
Bajpai M, Pande A, Tewari SK, Prakash D (2005) Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr 56:287–291
Siddhuraju P, Becker K (2003) Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem 51:2144–2155
Milton Prabu S, Sumedha NC (2014) Ameliorative effect of diallyl trisulphide on arsenic-induced oxidative stress in rat erythrocytes and DNA damage in lymphocytes. J Basic Clin Physiol Pharmacol 25(2):181–197
Shankar S, Singh G, Srivastava RK (2007) Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci 12:4839–4854
Kadirvel R, Sundaram K, Mani S et al (2007) Supplementation of ascorbic acid and α-tocopherol prevents arsenic-induced protein oxidation and DNA damage induced by arsenic in rats. Hum Exp Toxicol 26:939–946
Kelly SA, Havrilla CHM, Brady TC, Abramo KH, Levin ED (1998) Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ Health Perspect 106:375–384
Yamanaka K, Hasegawa A, Sawamura R, Okada S (1991) Cellular response to oxidative damage in lung induced by the administration of dimethylarsinic acid, a major metabolite of inorganic arsenics, in mice. Toxicol Appl Pharmacol 108:205–213
Acharyya N, Chattopadhyay S, Maiti S (2014) Chemoprevention against arsenic-induced mutagenic DNA breakage and apoptotic liver damage in rat via antioxidant and SOD1 upregulation by green tea (Camellia sinensis) which recovers broken DNA resulted from arsenic-H2O2 related in vitro oxidant stress. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 32(4):338–361. doi:10.1080/10590501.2014.967061
Aposhian HV, Zakharyan RA, Avram MD, Kopplin MJ, Wollenberg ML (2003) Oxidation and detoxification of trivalent arsenic species. Toxicol Appl Pharmacol 193:1–8
Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C (2005) Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 16:577–586
Afzal M, Afzal A, Jones A, Armstrong D (2002) A rapid method for the quantification of GSH and GSSG in biological samples. Methods Mol Biol 186:117–122
Manna P, Sinha M, Sil PC (2008) Arsenic-induced oxidative myocardial injury: protective role of arjunolic acid. Arch Toxicol 82:137–149
Aposhian HV (1989) Biochemical toxicology of arsenic. Rev Biochem Toxicol 10:265–99
Radabaugh TR, Aposhian HV (2000) Enzymatic reduction of arsenic compounds in mammalian systems: reduction of arsenate to arsenite by human liver arsenate reductase. Chem Res Toxicol 13:26–30
Exner R, Wessner B, Manhart N, Roth E (2000) Therapeutic potential of glutathione. Wien Klin Wochenschr 112:610–616
Hayakawa T, Kobayashi Y, Cui X, Hirano S (2005) A new metabolic pathway of arsenite: arsenite-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol 79(4):183–191
Eder E, Wacker M, Lutz U, Nair J, Fang X, Bartsch H, Beland FA, Schlatter J, Lutz WK (2006) Oxidative stress related DNA adducts in the liver of female rats fed with sunflower-, rapeseed-, olive- or coconut oil supplemented diets. Chem Biol Interact 159:81–89
Kalia K, Narula GD, Kannan GM, Flora SJS (2007) Effects of combined administration of captopril and DMSA on arsenite induced oxidative stress and blood and tissue arsenic concentration in rats. Comp Biochem Physiol C Toxicol Pharmacol 144(4):372–379
Bhadauria S, Flora SJS (2004) Arsenic induced inhibition of δ-aminolevulinate dehydratase activity in rat blood and its response to meso-2,3-dimercaptosuccinic acid and monoisoamyl DMSA. Biomed Environ Sci 17:101–108
Flora SJS, Saxena G, Mehta A (2007) Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of reactive oxygen species and intracellular Ca2+. J Pharmacol Exp Ther 322:108–116
Singh S, Rana SVS (2007) Amelioration of arsenic toxicity by L-ascorbic acid in laboratory rat. J Environ Biol 28(2):377–384
Sumedha NC, Milton Prabu S (2013) Arsenic induced oxidative hematotoxicity in rats and its protection by diallyl trisulfide. Int J Biol Pharm Res 4(7):507–515
Wang ZG, Rivi R, Delva L et al (1998) Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARα independent manner. Blood 92(5):1497–1504
Dong Z (2002) The molecular mechanisms of arsenic-induced cell transformation and apoptosis. Environ Health Perspect 110(Suppl 5):757–759
Kadirvel R, Muthuswamy A, Samuel S, Panneerselvam C (2005) Ascorbic acid and α-tocopherol as potent modulators of apoptosis on arsenic induced toxicity in rats. Toxicol Lett 156(2):297–306
Chen NY, Ma WY, Yang CS, Dong Z (2000) Inhibition of arsenite-induced apoptosis and AP-1 activity by epigallocatechin-3-gallate and theaflavins. J Environ Pathol Toxicol Oncol 19(3):287–295
Flora SJS (2002) Nutritional components modify metal absorption, toxic response and chelation therapy. J Nutr Environ Med 12(1):53–67
Mishra D, Gupta R, Pant SC, Kushwah P, Satish HT, Flora SJS (2009) Co-administration of monoisoamyl dimercaptosuccinic acid and Moringa oleifera seed powder protects arsenic-induced oxidative stress and metal distribution in mice. Toxicol Mech Methods 19(2):169–182
Flora SJS, Chouhan S, Kannan GM, Mittal M, Swarnakar H (2008) Combined administration of taurine and monoisoamyl DMSA protects arsenic induced oxidative injury in rats. Oxidative Med Cell Longev 1(1):39–45
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, M., Lalit, M. & Thakur, R. Natural Antioxidants Against Arsenic-Induced Genotoxicity. Biol Trace Elem Res 170, 84–93 (2016). https://doi.org/10.1007/s12011-015-0448-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0448-7