Abstract
Auxiliary enzymes participate in β-oxidation of unsaturated fatty acids. The objective of the study was to investigate the impact of a moderate zinc deficiency and a high intake of polyunsaturated fat on Δ3Δ2-enoyl-CoA isomerase (ECI) in the liver and other tissues. Five groups of eight weanling rats each were fed moderately zinc-deficient (ZD) or zinc-adequate (ZA) semisynthetic diets (7 or 50 mg Zn/kg) enriched with 22 % cocoa butter (CB) or 22 % safflower oil (SO) for 4 weeks: (1) ZD-CB, fed free choice; (2) ZA-CBR, ZA-CB diet fed in equivalent amounts consumed by the ZD-CB group; (3) ZD-SO, fed free choice; (4) ZA-SOR, ZA-SO diet fed in equivalent amounts consumed by the ZD-SO group; and (5) ZA-SO, fed free choice. Growth and Zn status markers were markedly reduced in the ZD groups. ECI activity in the liver of the animals fed the ZD- and ZA-SO diets were significantly higher (approximately 2- and 3-fold, respectively) as compared with the CB-fed animals, whereas activities in extrahepatic tissues (kidneys, heart, skeletal muscle, testes, adipose tissue) were not altered by dietary treatments. Transcript levels of the mitochondrial Eci gene in the liver did not significantly differ between ZD and ZA rats, but were 1.6-fold higher in the ZA-SO- than in the ZD-CB-fed animals (P < 0.05). It is concluded that diets enriched with safflower oil as a source high in linoleic acid induce markedly increased hepatic ECI activities and that a moderate Zn deficiency does not affect transcription of the mitochondrial Eci gene in the liver.
Similar content being viewed by others
Introduction
A lack of zinc (Zn) impairs cell division, differentiation, and growth in microorganisms, plants, and animals (see [1] for review). In young animals, well-known early signs of Zn depletion are depressed appetite and retarded growth. Recent nutritional studies indicate that marginally Zn-deficient diets enriched with sunflower or olive oil reduce food intake, growth rate, and Zn status of weanling rats to a greater extent than diets enriched with beef tallow [2, 3]. Since fat accounted for at least 50 % of the dietary energy intake, it can be assumed that the animals largely relied on fatty acid degradation to gain the energy necessary for maintenance and growth functions. Mitochondrial and peroxisomal β-oxidation of unsaturated fatty acids (UFA), mainly oleic, linoleic, and α-linolenic acid in vegetable oils, requires auxiliary enzymes for the removal of cis-double bonds of UFA. There are two enzymes catalyzing the cis–trans conversion of the double bonds of fatty acids, Δ3,Δ2-enoyl-CoA isomerase (ECI; EC 5.3.3.8) and NADPH-dependent 2,4-dienoyl-CoA reductase (DECR; EC 1.3.1.34) (see [4] for review). ECI converts 3-cis- and 3-trans-enoyl-CoA esters of UFA to 2-trans-enoyl-CoA esters, which are thus available for continued degradation in the β-oxidation cycle. DECR reduces double bonds at even-numbered positions to 3-enoyl-CoA esters, which then can be isomerized further by ECI. Hence, ECI plays a pivotal role as its activity is essential for the catabolism of all UFA. For the removal of double bonds at odd-numbered positions of fatty acids, an alternative, DECR-dependent pathway has been proposed, in which Δ3,Δ5-Δ2,Δ4-dienoyl-CoA isomerase (EC 5.5.5.-) participates [4]. In DECR-null mice, however, no intermediates for this route have been detected, suggesting that the degradation of these latter fatty acids proceeds quantitatively via a DECR-independent pathway [5]. High Eci expression levels have been reported for the liver, kidney, heart, and skeletal muscle [6], which are also known as important fat-burning organs. Previous studies found that the expression of the mitochondrial ECI gene (Eci1, synonym Dci; NBCI gene accession No. D00729/M61112) is reduced in the liver of Zn-deficient young rats [7, 8].
The objective of our study was to investigate the impact of a moderate dietary Zn deficiency and a high intake of polyunsaturated fatty acids (PUFA) on the activity of the mitochondrial ECI enzyme in the liver and extrahepatic tissues and on the expression level of the mitochondrial ECI gene in the liver of weanling rats. Safflower oil (SO) served as fat source rich in PUFA, predominantly linoleic acid, and cocoa butter (CB) as source rich in saturated fatty acids.
Experimental Methods
Animals and Experimental Treatments
Forty male weanling rats (Wistar, Harlan-Winkelmann, Borchen, Germany) with an initial live weight of 50.8 ± 0.2 g (mean ± SD) were randomly allocated to five groups of eight animals each. They were fed moderately zinc-deficient (ZD) or zinc-adequate (ZA) semi-synthetic diets (7 or 50 mg Zn/kg) enriched with 22 % cocoa butter (CB) or 22 % safflower oil (SO) for 4 weeks. The feeding protocol for these groups was as follows: (1) ZD-CB, fed the ZD-CB diet free choice; (2) ZA-CBR, fed restrictedly the ZA-CB diet in equivalent amounts consumed by the ZD-CB group on the previous day; (3) ZD-SO, fed the ZD-SO diet free choice; (4) ZA-SOR, fed restrictedly the ZA-SO diet in equivalent amounts consumed by the ZD-SO group on the previous day; and (5) ZA-SO, fed the ZA-SO diet free choice. Demineralized water was offered freely. Diets contained (g/kg) powdered egg albumin, 200; corn starch, 67; sucrose, 100; soybean oil, 30; l-lysine, 1.5; l-methionine, 1.5; cellulose, 280; CB or SO, 220; mineral mix, 70; vitamin premix, 10; and zinc premix, 20. Soybean oil was used as source of essential fatty acids, and cellulose was used as a diluent in order to restrict dietary energy density. The mineral mix was composed as described previously [9]. The vitamin premix, which contained corn starch as filler, supplied 40 mg dl-α-tocopheryl acetate and 1,100 mg choline chloride per kg diet and other vitamins as previously described [9]. The zinc premix supplied 7.0 or 50 mg zinc/kg diet as Zn sulfate and corn starch. By analysis (five replicates per diet), Zn concentrations of the ZD-CB, ZD-SO, ZA-CB, and ZA-SO diets averaged 7.4 (SD, 0.6), 7.4 (0.7), 50.0 (6.9), and 50.5 (3.1), respectively. Based on food tables [10], the metabolizable energy concentration of the diets was 15.7 MJ/kg. Fat and carbohydrates (starch and sucrose) contributed about 59 and 22 energy%, respectively. By analysis, UFA accounted for 45 and 90 % and linoleic acid for 11 and 76 % of the total fatty acids in the CB and SO diets, respectively (Fig. 1).
The animals were kept individually in metabolic cages (Macrolon, stainless-steel metal grids) under controlled environmental conditions (22 °C, 60 % relative humidity, 12-h light–dark cycle, lights on at 0700 hours). Food remnants were removed daily at 0900 hours and replaced by fresh portions. Urine was collected quantitatively during weeks 3 and 4 from five animals of each diet group and stored at −20 °C until analysis. After 4 weeks, food was withdrawn overnight (10–12 h) before the animals were anesthetized in a carbon dioxide atmosphere and decapitated. Blood was collected in heparinized tubes to obtain plasma. The liver, kidneys, heart, skeletal muscle (surrounding right femur), femur bone, testes, and abdominal adipose tissue were immediately removed from the carcasses and weighed. A segment of the central liver lobe was excised under sterile conditions and snap-frozen in liquid nitrogen for later RNA extraction. All tissues were stored at −80 °C. Care, handling, and all treatments of the rats followed established guidelines. Written approval was obtained by the Animal Protection Authority of the State (II 25.3-19c20/15c GI 19/3).
Analytical Methods
Zinc Status
Zn concentration in plasma was analyzed by hydride atomic absorption spectrometry (AAS; PU 9400, Phillips, Kassel, Germany) after dilution with 0.1 M HCl (1:20, v/v). Diet samples and femora were wet-ashed with 65 % (w/v) HNO3 and diluted with aqua bidest. for Zn analysis by ICP-AES (Type 701, Unicam). Accuracy of Zn analysis was checked by the inclusion of standard samples of known Zn content. The activity of the alkaline phosphatase in plasma was assayed in triplicate by a standard assay [11]. A control serum (Qualitrol, Merck, Darmstadt, Germany) was used for quality control. The metallothionein (MT) content of the liver was determined by the cadmium-binding assay [12]. Briefly, duplicate liver samples were homogenized in 10 mM Tris buffer, pH 7.4 (1:10, w/w) under nitrogen atmosphere and centrifuged for 10 min at 12,000 × g and 4 °C. An aliquot of the supernatant was mixed with a cadmium solution (containing 109Cd). Excess cadmium was removed by the addition of a hemoglobin solution, heating to 100 °C for 10 min, and centrifugation. MT concentration was expressed as nanomoles of MT per gram fresh weight, assuming a molecular weight of 6,000 Da and a binding of 6 mol Cd/mol MT.
Fatty Acid Pattern of Diets
Diet samples (duplicates) were heated in 0.4 M HCl and dried for lipid extraction by hexane (containing 0.005 %, w/v, butylhydroxytoluol). Heptadecanoic acid was added as internal standard to the extracts before transmethylation by the N-trimethylsulfonium hydroxide method [13]. Fatty acid methyl esters (FAME) were separated by gas chromatography (Chrompac 9400; 50 m Permabond FFAP-DF column, Machery and Nagel; flame ionization detector) and were identified by comparing retention times of FAME in a standard mix (Sigma 18919). Individual FA were quantified on the basis of area under the curve and internal standard response and expressed as weight percentage of the total amount of fatty acids.
Preparation of Mitochondria-Enriched Fractions
Frozen tissue sections (duplicates of 0.5 g) of the liver, kidneys, heart, skeletal muscle, and testes were homogenized in 50 mM Na phosphate buffer (pH 7.4; 1:5 w/w; +4 °C). Homogenates were centrifuged at 630 × g for 10 min to remove intact cells, nuclei, and cell debris. The sediment was washed with phosphate buffer. Combined supernatants were centrifuged at 13,000 × g for 15 min. Sediments were rinsed twice with phosphate buffer. These mitochondria-enriched fractions were dissolved in phosphate buffer, split into aliquots, and stored (−80 °C) for subsequent enzyme and protein analyses. In the case of the abdominal adipose tissue, 0.7 g sections were used. After homogenization and centrifugation, supernatant was carefully removed from beneath the top fat layer.
Δ3,Δ2-Enoyl-CoA Isomerase
For the determination of the activity of the ECI (EC 5.3.3.8), trans-3-hexenoyl-CoA was used as substrate. The trans-3-hexenoyl-CoA was synthesized from trans-3-hexenoic acid (Aldrich, W31,700-4) and coenzyme A (Gerbu, 1094) following the procedural steps described previously [14, 15]. Before its use in ECI assays, impurities were removed by reversed-phase high-performance liquid chromatography (HPLC) (Merck Hitachi system) with a LiChrospher 100 RP-18-column (5 μm, 250 × 4 mm; Merck) by modifying the method of Zhang et al. [16]. HPLC analysis of the purified substrate showed only a single peak accounting for 99.3 % of the area under the curve. In addition, the purity of the trans-3-hexenoyl-CoA was verified by ESI mass spectra (LCQ, Thermo Finnigan MAT). For the ECI assays, a stock solution of trans-3-hexenoyl-CoA was prepared (0.7 mM). Substrate concentration was determined by the procedure of Ellman [17], and its final concentration in ECI assays was rechecked at 260 nm (extinction coefficient 13.7 mM−1 cm−1) [18].
ECI activity was determined as described by Stoffel and Ecker [19]. Tissue fractions were heated to 70 °C for 120 s to inactivate the heat-sensitive enoyl-CoA-hydratase (EC 4.2.1.17) and then cooled on ice. Precipitated protein was removed by centrifugation (15 min, 13,000 × g). ECI activity was measured in the clear supernatant in a semi-micro scale. In one-way UV cuvettes, 50 μL trans-3-hexenoyl-CoA (0.7 mM), 50 μL BSA solution (fraction V, fatty acid free, 0.1 %, w/v) and 380 μL 50 mM Na phosphate buffer (pH 7.4) were combined, carefully mixed and incubated (25 °C). After the addition of 20 μL of the tissue sample, the increase in absorbance was measured at 263 nm for 2 min (Cary 50 Bio, Varian). Linearity was observed in the range of 0.01 to 0.04 U/min (r = 0.995). The coefficient of variance (CV) of ten replicate measurements in the linear range was 4.0 %; that of six separate preparations of liver tissue was 5.7 %. The ECI activity was assessed in triplicate and expressed in units per milligram protein. The specificity of the reaction was verified by adding enoyl-CoA hydratase to the assay mixtures, which resulted in a return of the extinction to its initial value.
Succinate Dehydrogenase
The activity of succinate dehydrogenase (SDH; EC 1.3.5.1), an integral protein of the inner mitochondrial membrane, was determined as a marker enzyme. Its activity is not affected by Zn deficiency [20]. Also, dietary fat source (palm, safflower, or perilla oil) did not alter the specific activity of SDH in liver mitochondria of growing rats [21]. The SDH activity was measured in triplicate by the method of Veeger et al. [22]. Reproducibility was assessed with liver samples (CV = 4.1 % for six separate tissue preparations; CV = 2.1 % for ten repeat assays of a liver sample).
Protein Concentration
Protein concentration in tissue homogenates used for the determination of enzyme activities was determined in triplicate by the method of Bradford [23], using bovine serum albumin for calibration. The extinction was measured at 595 nm after 10 min.
3-Hydroxybutyrate
The concentrations of 3-hydroxybutyrate (3-HB) in plasma and urine of five animals of each group were determined in duplicate by a commercial kit (Autokit 3-HB; Wako Chemicals GmbH, Neuss, Germany).
Expression of the ECI Gene in the Liver
Total RNA content was extracted from pooled liver samples (50 mg from two animals each; three separate pools per diet group) by the acid guanidinium thiocyanate–phenol–chloroform procedure [24] under RNase-free conditions. Final RNA pellets were washed twice with 70 % (v/v) ethanol, vacuum-dried, dissolved in H2O-diethylpyrocarbonate (DEPC) solution, and stored in portions at −80 °C. RNA concentrations were assessed spectrometrically at 260 and 280 nm against H2O-DEPC. Purity of RNA preparations was checked by gel electrophoresis and ethidium bromide staining. Reverse transcription of the harvested liver RNA was performed by a commercial kit using the oligo(dT)18-primer (RevertAIDTM First Strand cDNA Synthesis Kit, Fermentas GmbH, St Leon-Rot, Germany), following the procedural steps of the manufacturer’s instructions. The first-strand cDNA was amplified in volumes of 50 μL, which contained 10× PCR buffer in 20 mM MgCl2 (Fermentas), 2 mM dNTP mix (Fermentas), Taq polymerase (5 U/μL; Peqlab), specific forward and reverse primers (10 μM; MWG Biotech-AG), and H2O-DEPC. The primers were for the mitochondrial short-chain ECI (Eci1; accession number X61184, NCBI): forward 5′-CAGGATAATG GCGGACAACT-3′, reverse 5′-TACACGTGCAGGGACTTCTG-3′ and for glycerol aldehyde-3-phosphate dehydrogenase (Gapdh; NM_017008, NCBI) as housekeeping gene: forward, 5′-ACGGGAAGCTCACTGGCATG-3′, reverse, 5′-CCACCACCCTGTTGCTGTAG-3′. The cDNA (about 1 μg in 2 μL) was amplified in 26 to 32 cycles (MyCycler, Bio-Rad Laboratories Inc.). PCR products were electrophoresed on 1.5 % ethidium bromide agarose gel. A base pair standard (GeneRuler DNA Ladder Plus, Fermentas) was included to check the length of the fragments. Spots were recorded by a Chemi Imager equipped with a CCD video system and Alpha Ease Imaging Software (Central Biotechnology Unit of the Justus Liebig University, Giessen, Germany). The digital photographs were analyzed by the software package Gelscan 5.1 (BioSciTec GmbH, Frankfurt am Main, Germany). The expression of Eci1 was quantified as the background-corrected intensity relative to the intensity recorded for Gapdh, which was used to normalize the Eci1-mRNA levels. Final data of Eci1-mRNA were expressed as a multiple of the lowest mean value among diet groups.
Statistical Analyses
Results were analyzed by one-way analysis of variance (ANOVA), using the IBM SPSS package, version 19 for windows. Homogeneity of variance was checked by the Levene test. In the case of heterogeneity of variance, data were logarithmically transformed. The Tukey HSD procedure was applied for post hoc comparisons among the five groups (significance level set at P < 0.05). Standard errors of the mean (SEM) are based on the residual error of one-way ANOVA.
Results
Food Intake, Growth, and Zinc Status
Food intake and body weight gain of the weanling rats fed the ZD-CB-and ZD-SO diets were significantly lower (P < 0.05) than those recorded for the animals fed the ZA-SO diet free choice (Table 1). But food intake and body weight gain were reduced to a greater extent in the ZD-SO than in the ZD-CB group (P < 0.05). Weight gains of the animals in the ZA-CBR and ZA-SOR groups were comparable to those consuming the respective Zn-deficient diets free choice. The relative liver weights of the animals offered the ZD diets were about 5 to 12 % lower (P < 0.05) than for those consuming the ZA diets.
Plasma and femur Zn concentrations were significantly reduced in the rats fed the ZD diets as compared to those fed the ZA diets (P < 0.05; Table 2 ), whereas liver Zn concentrations remained closely comparable among all diet groups. Plasma and femur Zn concentrations in the ZD-SO group were approximately 36 and 24 % lower, respectively, than in the ZD-CB group (P < 0.05). The ZD diets markedly lowered plasma AP activities and liver metallothionein (MT) concentrations. The ZA-SO group displayed the highest MT concentration among all groups (P < 0.05).
Activity of the Δ3,Δ2-Enoyl-CoA Isomerase and Succinate Dehydrogenase
The activity of the ECI and SDH and their ratio (ECI/SDH) in the liver were comparable among the ZD- and ZA-CB groups, regardless of the marked difference in dietary Zn level (Table 3). The hepatic ECI activity in the rats fed the SO-based diets was approximately 2- to 3-fold higher (P < 0.05) than in those consuming the two CB-based diets. In the ZD-SO group, however, the ECI activity was at least one quarter lower than in the ZA-SOR and ZA-SO groups (P < 0.05). In contrast, the SDH activity did not significantly differ among diet groups (P > 0.05; Tukey test). Accordingly, the ECI/SDH ratios vary among diet groups in a very similar pattern as the ECI activities.
In the kidneys, heart, skeletal muscle, testes, and abdominal adipose tissue, ECI and SDH activities, likewise ECI/SDH activity ratios, were not significantly affected by dietary Zn supply nor by fat source (data not shown). The overall mean activities in these tissues are shown in Table 4, along with statistical traits. The significant P value (P = 0.029) in the case of the ECI activity in the heart is due to a significant difference between the ZA-SOR and ZA-SO groups (data not shown). The highest ECI activities among the extrahepatic tissues were observed in kidneys and heart.
Expression of the Mitochondrial Δ3,Δ2-Enoyl-CoA Isomerase in the Liver
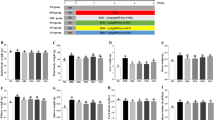
The relative mRNA-Eci1 levels in the liver did not significantly (P > 0.05) differ between the groups fed the ZD and ZA diets (Fig. 2). There was a 1.6-fold difference in mRNA-Eci1 abundance between the ZD-CB and the ZA-SO group (P < 0.05).
mRNA expression of Δ3,Δ2-enoyl-CoA isomerase (Eci) in the liver. a Ethidium bromide fluorescence of the RT-PCR amplifications of Eci and Gapdh; b relative expression of Eci-mRNA (see “Experimental Methods” section). ZD-CB 7 mg Zn/kg diet with 22 % cocoa butter fed free choice, ZA-CBR 50 mg Zn/kg diet with 22 % cocoa butter fed in the equivalent amounts consumed by the ZD-CB group, ZD-SO 7 mg Zn/kg diet with 22 % safflower oil fed free choice, ZA-SOR 50 mg Zn/kg diet with 22 % safflower oil fed in the equivalent amounts consumed by the ZD-SO group, ZA-SO 50 mg Zn/kg diet with 22 % safflower oil fed free choice. a,b,cMeans (± SEM, n = 3/diet group) not sharing common superscript letters within columns significantly differ (P < 0.05; Tukey test)
Plasma Concentration and Renal Excretion of 3-Hydroxybutyrate
The rats that had free access to their respective diet displayed approximately 2-fold higher plasma 3-HB concentrations than those of the ZA-CBR and ZA-SOR groups exposed to food restriction (P < 0.001; Table 5). In contrast, renal excretion of 3-HB during weeks 3 and 4 of the experimental period did not significantly differ among these groups.
Discussion
Dietary fatty acids are an important energy source for the liver and extrahepatic tissues, especially when glucose is limited. beta-Oxidation of unsaturated fatty acids requires auxiliary enzymes, among which ECI plays a pivotal role [4]. Here we investigated the effect of a moderate Zn deficiency on the activity of ECI in the liver and extrahepatic tissues and on the expression of the Eci1 gene in the liver of weanling rats exposed to low-carbohydrate diets in which unsaturated fatty acids, primarily linoleic acid, were the major energy source. As expected, the rats fed the ZD diets showed a markedly diminished Zn status evident from pronounced reductions in food intake, body weight gain, plasma and femur Zn concentrations, plasma alkaline phosphatase activity, and liver MT concentration as compared to the values recorded for the animals fed the ZA diets. Remarkably, the ZD-SO diet was associated with a greater decrease in food consumption and growth than the ZD-CB diet despite the same dietary Zn level. This difference, however, cannot simply be attributed to a lower palatability of the safflower oil-based diet for several reasons. First, plasma and femur Zn concentrations indicate a poorer Zn status of the rats fed the ZD-SO diet as compared to those offered the ZD-CB diet (Table 2). These Zn status markers are not affected by differences in food consumption as is evident from the values in the ZA-SOR group compared to those in the ZA-SO group, in agreement with previous reports [25, 26]. Secondly, moderately Zn-deficient diets enriched with sunflower oil or olive oil have also been found to reduce food intake, growth, and Zn status of young rats more markedly than diets enriched with beef tallow or other saturated fats, whereas there was no difference in food intake and growth when these animals were fed corresponding Zn-adequate diets [2, 3]. In a study in which young rats could freely select between two diets containing either 7 % lard or fish oil [27], intake of the fish-oil diet decreased as the dietary Zn level was reduced from an adequate to a marginally deficient to a severely deficient level (30.9, 5.9, and 0.9 mg Zn/kg diet, respectively). Finally, the loss of appetite of Zn-depleted animals cannot be considered as the primary cause of growth retardation. Force-feeding young rats Zn-depleted diets above appetite does not improve growth but instead quickly elicit severe ill health and morbidity [25, 28]. Taken together, the present experiment supports former studies [2, 3, 27] finding that Zn-deficient diets rich in unsaturated fats adversely affect growth and Zn status of young rats. The reason for this effect is not known.
The ECI activity measured in the present experiment comprises substrate (trans-3-hexenoyl-CoA) conversion by all isoenzymes, namely mitochondrial short-chain ECI (MECI), mitochondrial and peroxisomal monofunctional ECI, and the peroxisomal multifunctional ECI (MFE1) [16]. Almost all isomerase activity is associated with the mitochondrial fraction in rat liver [29]. MECI displays a much higher catalytic efficiency toward trans-3-hexenoyl-CoA as substrate and a lower chain-length dependence than the other isoenzymes [16]. Therefore, it can be assumed that the hepatic ECI activity recorded in our study, for the major part, is due to MECI, the predominant enzyme for isomerization.
In previous studies [30–32], the activity of the auxiliary enzymes of β-oxidation, ECI and DECR, was elevated in the liver of rats fed diets containing 15 % oils rich in PUFA as compared to palm oil. In these studies, safflower oil did not raise the hepatic activity of the auxiliary enzymes as consistently and effectively as oils rich in 18:3n-6 (borage oil), 18:3n-3 (linseed or perilla oil), or fish oil did. In our experiment, the specific ECI activity in the liver of the three groups fed the SO diets was 2.3–3.2-fold higher than in the two groups fed the CB diets. This pronounced effect of fat source, which is in line with the expected biological response to the 2-fold higher UFA content in the SO diets than in the CB diets, is greater than the difference in the hepatic ECI activity observed previously between safflower- and palm oil-based diets in young rats [30–32].
The hepatic ECI activity in the rats fed the ZD-SO diet was at least one quarter lower (P < 0.05) than in the liver of the animals offered the ZA-SO diet either free choice or in restricted amounts, whereas it was comparable between the two CB groups despite the difference in dietary Zn level (Table 3). This difference suggests an interaction between the dietary Zn supply and fat source and possibly indicates that the deficient Zn intake in the ZD-SO group prevented the enzyme activity to rise to the higher level attained in the ZA-SOR and ZA-SO groups. Evidently, the amount of food consumed was of no importance, since the latter two groups displayed similar hepatic ECI activities. The literature does not indicate that ECI is a Zn-dependent metalloenzyme, in contrast to alkaline phosphatase, which served as a Zn status marker at the protein level in our study. ECI, as in the case of the vast majority of the mitochondrial proteins, is synthesized as a precursor protein in the cytoplasm of the cell [33]. After its transport into mitochondria, its leader amino acid sequence is removed in a two-step proteolysis by a general mitochondrial processing peptidase (EC 3.4.24.64) and the mitochondrial intermediate peptidase (MIP; EC 3.4.24.59). There is evidence that MIP is a metallopeptidase containing a highly conserved putative Zn-binding domain [34]. It might be expected, however, that an inhibition of MIP function due to Zn depletion would also affect the activity of other mitochondrial proteins, including SDH. SDH activities, however, were not reduced by the marginal Zn deficit in our study, nor were hepatic Zn concentrations. Further research is needed to elucidate the relationship between the hepatic ECI enzyme(s) and Zn nutrition.
Diets containing perilla or fish oil, known to be rich in n-3 PUFA, have been found to markedly enhance the transcription of the Eci1 gene in the liver of rodents [32, 35]. Hepatic Eci1-mRNA abundance also increased when rodents were fed diets enriched with linoleic acid as compared with saturated fatty acids, but the differences were not significant [32, 35, 36]. In our study, hepatic Eci1 transcript levels significantly differed only between the ZD-CB and the ZA-SO groups (1.6-fold; Tukey test), which also expressed the lowest and highest ECI activities, respectively. Between these two groups, intake of UFA differed to the greatest extent, approximately 7.7-fold in the case of linoleic acid. Overall, hepatic Eci1 transcript levels and ECI activities display a similar pattern among the five diet groups (r = 0.64; P = 0.015, n = 15), although the relative differences in Eci1-mRNA abundance among treatment groups are smaller than those observed for the enzyme activities.
Previous studies [7, 8] reported that transcript levels of Eci1 (synonym Dci) and other genes coding for proteins involved in fatty acid catabolism were down-regulated in the liver of Zn-deficient young rats as compared to Zn-adequate control animals, whereas transcript levels of genes involved in fatty acid synthesis were up-regulated. In contrast, we found that the relative Eci1-mRNA abundance was not reduced in the rats fed the ZD diets as compared to those fed the ZA-SO diet free choice. Diet composition, in particular zinc, carbohydrate, and fat content, and feeding regime markedly differ between our and the previous studies [7, 8]. In our experiment, Zn depletion of the rats fed the ZD diets was moderate, allowing considerable growth, as discussed above, and fat contributed nearly 60 energy%, whereas carbohydrates (starch and sucrose) accounted for no more than about 22 energy%. Hence, it is reasonable to assume that hepatic de novo synthesis of fatty acids was largely depressed in our experiment. In support, the activity of glucose-6-phosphate dehydrogenase, a lipogenic enzyme that correlates with the rate of de novo fatty acid synthesis [37], was greatly reduced in the liver of growing rats fed similar low-carbohydrate, high-fat diets as compared to the hepatic activity in animals fed a low-fat, high-carbohydrate diet [3]. The most prominent difference in the experimental protocols concerns the feeding regimen. In the former studies [7, 8], the animals were fed by intragastric tube in order to equalize quantity and frequency of intake of the severely Zn-deficient and Zn-adequate diets. The Zn-deficient diet was fed in excess of amounts that are freely consumed by Zn-depleted young rats [38], whereas the equivalent amount of the Zn-adequate diet fed to the control animals was below the expected intake in a free-choice feeding regimen and limited growth rate to merely 2.4 g/day [7], which is appreciably below the weight gains attained by the rats consuming the ZD and ZA diets in our experiment at a similar body weight. Forced overfeeding can be expected to induce lipogenesis and fatty livers and induce down-regulation of genes coding for enzymes of fatty acid catabolism [39]. Accordingly, the livers of young rats force-fed Zn-deficient diets show increased activities of lipogenic enzymes [40] and higher triglyceride concentrations [8, 38]. In contrast, triglyceride concentrations in the liver of rats fed Zn-deficient diets free choice are not higher than in ad libitum- or pair-fed Zn-adequate controls [38]. Therefore, it seems likely that the previously observed difference in the hepatic Eci1 transcript levels between force-fed Zn-deficient and Zn-adequate rats [7, 8] was merely the consequence of opposing metabolic effects of over- and underfeeding, respectively, and not due to a deficit of zinc as such.
Considering that ECI is essential for a complete degradation of UFA and that the ECI activity in the liver appreciably differed between the rats fed the ZD-SO diet and those fed the ZA-SO diet either free choice or in reduced amounts, it can be asked whether a deficit of zinc is apt to adversely affect fatty acid catabolism, especially when UFA are the preponderant energy source as it was the case for the animals fed the SO-based diets. Metabolic evidence of our study and previous work indicates that dietary Zn depletion does not impair β-oxidation of fatty acids and hepatic ketogenesis [41–45]. In our experiment, renal excretion of 3-HB during the last 2 weeks did not differ among the five diet groups. Plasma 3-HB concentrations of the rats fed the ZD-SO diet were comparable to those of the animals fed the ZD-CB and ZA-SO diets free choice, suggesting that the lower hepatic ECI activity of the former animals did not limit the increased demand for β-oxidation and ketogenesis in response to the overnight fasting period. The 2-fold lower plasma 3-HB concentrations in the ZA-CBR and ZA-SOR groups may be attributed to the chronic food restriction. This is consistent with a previous study in which fasting plasma ketone body concentrations of Zn-depleted young rats were nearly three times as high as in Zn-adequate pair-fed animals [41]. In severely Zn-deficient young rats, the oxidation of palmitic acid was not reduced 14 h after food withdrawal as compared to Zn-adequate animals [42]. Whole-body oxidation of linoleic and α-linolenic acid, and serum 3-HB concentrations in Zn-deficient rats were higher than in control animals [43, 44]. Pregnant rats that were fed a suboptimal zinc diet (6 μg Zn/g diet) showed a substantially higher plasma 3-HB concentration and oxidation of 3-HB than Zn-sufficient control animals [45], which indirectly reflects a corresponding difference in β-oxidation of fatty acids. Both groups were in late gestation and in a negative energy balance without evidence of maternal hypoglycemia.
Mitochondrial β-oxidation of fatty acids is the major route of supplying energy in various extrahepatic tissues. The SDH activities recorded in the kidneys, heart, and skeletal muscle in the present study exceeded those in the liver, reflecting a high mitochondrial density. These tissues, in particular the kidneys, heart, and testes, also expressed relatively high ECI activities and ECI/SDH ratios compared to those in the liver, suggesting a high capacity to isomerize UFA. High Eci expression levels in the liver, kidney, heart, and skeletal muscle have been observed previously [6]. In our study, ECI activities in extrahepatic tissues were neither influenced by the mild dietary Zn deficit nor by the fat source (data not shown). This lack of response may suggest a constitutive expression of ECI at the protein level that is sufficiently high to isomerize a profound load of UFA, even under the conditions of a mild Zn deficit.
In conclusion, our study indicates that a moderate dietary Zn deficiency does not reduce mRNA abundance of the mitochondrial short-chain ECI in the liver of weanling rats fed low-carbohydrate diets enriched with 22 % CB or SO. Feeding of the SO-based diets was associated with notably increased activities of ECI in the liver, but to a significantly lesser extent in the rats fed the ZD-SO diet, whereas ECI activities in extrahepatic tissues were not altered by dietary treatments. In line with previous research, renal excretion and plasma levels of 3-HB suggest that β-oxidation of linoleic acid, the major dietary fatty acid in the SO-based diets, was not affected by the marginal Zn deficiency.
References
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Weigand E (2012) Fat source affects growth of weanling rats fed high-fat diets low in zinc. J Anim Physiol Anim Nutr 96:17–24
Weigand E, Boesch-Saadatmandi C (2012) Interaction between marginal zinc and high fat supply on lipid metabolism and growth of weanling rats. Lipids 47:291–302
Hiltunen JK, Qin Y-M (2000) β-Oxidation—strategies for the metabolism of a wide variety of acyl-CoA esters. Biochim Biophys Acta 1484:117–128
Miinalainen IJ, Schmitz W, Huotari A, Autio KJ, Soininen R et al (2009) Mitochondrial 2,4-dienoyl-CoA reductase deficiency in mice results in severe hypoglycemia with stress intolerance and unimpaired ketogenesis. PLoS Genet 5(7):e1000543. doi:10.1371/journal.pgen.1000543
Müller-Newen G, Stoffel W (1991) Mitochondrial 3-2trans-enoyl-CoA isomerase. Purification, cloning, expression, mitochondrial import of the key enzyme of unsaturated fatty acid β-oxidation. Biol Chem Hoppe-Seyler 372:613–624
tom Dieck H, Döring F, Roth H-P, Daniel H (2003) Changes in rat hepatic gene expression in response to zinc deficiency as assessed by DNA arrays. J Nutr 133:1004–1010
tom Dieck H, Döring F, Fuchs D, Roth H-P, Daniel H (2005) Transcriptome and proteome analysis identifies the pathways that increase hepatic lipid accumulation in zinc-deficient rats. J Nutr 135:199–205
Boesch-Saadatmandi C, Most E, Weigand E (2007) Influence of dietary fat and zinc supplementation on the iron utilization in growing rats. Ann Nutr Metabol 51:395–401
Souci SW, Fachmann W, Kraut H (eds) (2012) Food composition and nutrition tables, 7th rev edn. Medpharm Scientific, Stuttgart
Deutsche Gesellschaft für Klinische Chemie (1972) Standardisierung von Methoden zur Bestimmung von Enzymaktivitäten in biologischen Flüssigkeiten. Z Klin Chem Klin Biochem 8:658–660
Eaton DL, Toal BF (1982) Evaluation of the Cd/hemoglobin affinity assay for the rapid determination of metallothionein in biological tissues. Toxicol Appl Pharmacol 66:134–142
Syhre M, Hanschmann G, Heber R (1996) Derivatisierungstechniken in der Rückstandanalytik. GIT Fachz Labor 11:1121–1128
Stoffel W, Caesar H, Ditzer R (1964) The metabolism of unsaturated fatty acids. IV. On the beta-oxidation of mono-and polyene-fatty acids. Chemical syntheses of intermediary products. Hoppe-Seyler’s Z Physiol Chem 339:182–193
Fong JC, Schulz H (2002) Short-chain and long-chain enoyl-CoA hydratases from pig heart muscle. Methods Enzymol 71Pt C:390–398
Zhang D, Yu W, Geisbrecht BV, Gould SJ, Sprecher H, Schulz H (2002) Functional characterization of Δ3, Δ2-enoyl-CoA isomerases from rat liver. J Biol Chem 277:9127–9132
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Lawson LD, Kummerow F (1979) beta-Oxidation of the coenzyme A esters of elaidic, oleic, and stearic acids and their full-cycle intermediates by rat heart mitochondria. Biochim Biophys Acta 573:245–254
Stoffel W, Ecker W (1969) Delta3-cis, delta2-trans-enoyl-CoA isomerase from rat liver mitochondria. Methods Enzymol 14:99–105
Prasad AS, Oberleas D, Wolf P, Horwitz HP (1967) Studies on zinc deficiency: changes in trace elements and enzyme activities in tissues of zinc-deficient rats. J Clin Invest 46:549–557
Ide T, Murata M, Sugano M (1996) Stimulation of the activities of hepatic fatty acid oxidation enzymes by dietary fat rich in α-linolenic acid in rats. J Lipid Res 37:448–463
Veeger C, Dervartanian DV, Zeylemaker WP (1969) Succinate dehydrogenase. Methods Enzymol 13:81–90
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162:156–159
Park JH, Grandjean CJ, Antonson DL, Vanderhoof JA (1986) Effects of isolated zinc deficiency on the composition of skeletal muscle, liver and bone during growth in rats. J Nutr 116:610–617
Koo SI, Lee CC (1989) Effect of marginal zinc deficiency on lipoprotein lipase activities in postheparin plasma, skeletal muscle and adipose tissues in the rat. Lipids 24:132–136
Sato A, Nakashima Y (2011) Rats allowed to self-select zinc-deficient lard and fish-oil diets did not develop a preference for fish-oil diet. J Nutr Sci Vitaminol (Tokyo) 57:156–161
Chesters JK, Quarterman J (1970) Effects of zinc deficiency on food intake and feeding pattern of rats. Br J Nutr 24:1061–1069
Li D, Wong C-K, Yu W, Li P (2002) Cloning expression, and purification of the functional Δ3-Δ2-enoyl-CoA isomerase fusion protein. Protein Expr Purif 26:35–41
Kabir Y, Ide T (1996) Activity of hepatic fatty acid oxidation enzymes in rats fed alpha-linolenic acid. Biochim Biophys Acta 1304:105–119
Kumamoto T, Ide T (1998) Comparative effects of alpha- and gamma-linolenic acids on rat liver fatty acid oxidation. Lipids 33:647–654
Ide T, Kobayashi H, Ashakumary L, Rouyer IA, Takahashi Y, Aoyma T, Hashimoto T, Mizugaki M (2000) Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. Biochim Biophys Acta 1485:23–35
Gakh O, Cavadini P, Isaya G (2002) Mitochondrial processing peptidases. Biochim Biophys Acta 1592:63–77
Chew A, Rollins RA, Sakati WR, Isaya G (1996) Mutations in a putative zinc-binding domain inactivate the mitochondrial intermediate peptidase. Biochem Biophys Res Commun 226:822–829
Martin PGP, Guillou H, Lasserre F, Déjean S, Lan A, Pascussi J-M, SanCristobal M, Legrand P, Besse P, Pineau T (2007) Novel aspects of PPARα-mediated regulation of lipid and xenobiotic metabolism revealed through a nutrigenomic study. Hepatology 45:767–777
Takahashi Y, Kushiro M, Shinohara K, Ide T (2003) Activity and mRNA levels of enzymes involved in hepatic fatty acid synthesis and oxidation in mice fed conjugated linoleic acid. Biochim Biophys Acta 1631:265–273
Salati SM, Amir-Ahmady B (2001) Dietary regulation of expression of glucose-6-phosphate dehydrogenase. Annu Rev Nutr 21:121–140
Eder K, Kirchgessner M (1996) Effects of zinc deficiency on concentrations of lipids in liver and plasma of rats. Trace Elem Electrolytes 13:60–65
Desvergne B, Michalik L, Wahli W (2006) Transcriptional regulation of metabolism. Physiol Rev 86:465–514
Eder K, Kirchgessner M (1995) Zinc deficiency and activities of lipogenic and glycolytic enzymes in liver of rats fed coconut oil or linseed oil. Lipids 30:63–69
Quarterman J, Florence E (1972) Observations on glucose tolerance and plasma levels of free fatty acids and insulin in the zinc-deficient rat. Br J Nutr 28:75–79
Theuer RC, Hoekstra WG (1966) Oxidation of 14C-labeled carbohydrate, fat and amino acid substrates by zinc-deficient rats. J Nutr 89:448–454
Cunnane SC, Yang J, Chen Z-Y (1993) Low zinc intake increases apparent oxidation of linoleic and α-linolenic acids in the pregnant rat. Can J Physiol Pharmacol 71:205–210
Cunnane SC, Yang J (1995) Zinc deficiency impairs whole body accumulation of polyunsaturates and increases the utilization of [1-14C]linoleate for de novo lipid synthesis in pregnant rats. Can J Physiol Pharmacol 73:1246–1252
Greeley S, Sandstead HH (1983) Oxidation of alanine and β-hydroxybutyrate in late gestation by zinc-restricted rats. J Nutr 113:1803–1810
Acknowledgments
We gratefully acknowledge the GC–MS analysis of the trans-hexenoyl-CoA preparation in the laboratory of Prof. Dr. Hans Brückner, Institute of Nutritional Science, Justus Liebig University, Giessen. We are also grateful to Prof. Dr. Andreas Müller for his advice in PCR methodology, to Dr. Erika Most for her assistance in GC and HPLC analyses, and to Prof. Dr. Josef Pallauf and to the ZBB unit for providing access to laboratory instruments.
Conflict of Interest
The authors declare that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Justus, J., Weigand, E. The Effect of a Moderate Zinc Deficiency and Dietary Fat Source on the Activity and Expression of the Δ3Δ2-Enoyl-CoA Isomerase in the Liver of Growing Rats. Biol Trace Elem Res 158, 365–375 (2014). https://doi.org/10.1007/s12011-014-9940-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-9940-8