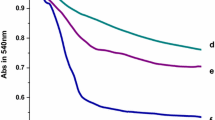
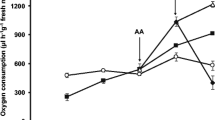
Abstract
Mitochondria play an important role in plant growth and development, cooperating with the endoplasmic reticulum and nucleus. Gadolinium, one of the rare earth elements, is an inhibitor of stretch-activated calcium channels located on the endoplasmic reticulum and plasma membrane and has no effect on nuclear calcium variation in plant cells. We analyzed the effects of Gd3+ on mitochondria function by monitoring mitochondrial swelling, changes of membrane fluidity, and transmembrane potential collapse and by observing mitochondrial ultrastructure. We found that high concentration of Gd3+ induces rice mitochondrial dysfunction through mitochondrial permeability transition (MPT). The protection of DTT and EDTA demonstrate that Gd3+ blocks the inner membrane ion channel through thiol chelation.







Similar content being viewed by others
References
Idée JM, Port M, Raynal I, Schaefer M, LeGreneur S, Corot C (2006) Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol 20:563–576
Bartolini ME, Pekar J, Chettle DR, McNeill F, Scott A, Sykes J, Prato FS, Moran GR (2003) An investigation of the toxicity of gadolinium based MRI contrast agents using neutron activation analysis. Magn Reson Imaging 21:541–544
Serkan K, Michael B (2003) Contrasting behaviour of anthropogenic gadolinium and natural rare earth elements in estuaries and the gadolinium input. Earth Planet Sci Lett 260:361–371
Brambilla S, Valaperta S, Graziani G, Montanelli A (2008) Gadolinium and lanthanum: a iatrogenic transmetallation? Clin Biochem 41:1029–1033
Bau M, Dulski P (1996) Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet Sci Lett 143:245–255
Zare-Dorabei R, Norouzi P, Ganjali MR (2009) Design of a novel optical sensor for determination of trace gadolinium. J Hazard Mater 171:601–605
Liu HX, Yuan L, Yang XD, Wang K (2003) La3+, Gd3+ and Yb3+ induced changes in mitochondrial structure membrane permeability, cytochrome c release and intracellular ROS level. Chem Biol Interact 146:27–37
Ye LH, Shi Z, Liu HX, Yang XD, Wang K (2010) Gadolinium induced apoptosis of human embryo liver L02 cell line by ROS-mediated AIF pathway. J Rare Earths 29:178–184
Zhu HD, Wu Q, Fan TJ, Liu QF, Zhang W (2008) Elemental transfer from Chinese soil via the diet to the whole human body. J Radiol Prot 28:573–580
Tyler G (2004) Rare earth elements in soil and plant systems—a review. Plant Soil 267:191–206
Curtis MJ, Wolpert TJ (2002) The oat mitochondrial permeability transition and its implication in victorin binding and induced cell death. The Plant Journal 29:295–312
Ding JP, Pickard BG (1993) Mechanosensory calcium-selective cation channels in epidermal cells. Plant J 3:83–110
Klűsener B, Boheim G, Liss H, Engelberth J, Weiler EW (1995) Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J 14:2708–2714
Xiong TC, Jauneau A, Ranjeva R, Mazars C (2004) Isolated plant nuclei as mechanical and thermal sensors involved in calcium signalling. The Plant Journal 40:12–21
Maurino VG, Peterhansel C (2010) Photorespiration: current status and approaches for metabolic engineering. Curr Opin Plant Biol 13:249–256
Yoshida K, Terashima I, Noguchi K (2011) How and why does mitochondrial respiratory chain respond to light? Plant Signal Behavx 6:864–866
Xia CF, Jin JC, Yuan L, Zhao J, Chen XY, Jiang FL, Qin CQ, Dai J, Liu Y (2013) Microcalorimetric studies of the effect of cerium (III) on isolated rice mitochondria fed by pyruvate. Chemosphere 91:1577–1582
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629
Vianello F, Macri E, Braidot EN, Mokhova EN (1995) Effect of 6-ketocholestanol on FCCP- and DNP-induced uncoupling in plant mitochondria. FEBS Lett 365:7–9
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Bioenerg Biomembr 177:751–766
Xia CF, Zhao J, Jin JC, Yuan L, Chen XY, Peng W, Jiang FL, Qin CQ, Dai J, Liu Y (2013) Ce(III)-induced rice mitochondrial permeability transition investigated by spectroscopic and microscopic studies. Biol Trace Elem Res 152:284–291
Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM (2003) Chenodeoxycholate induction of mitochondrial permeability transition pore is associated with increased membrane fluidity and cytochrome c release: protective role of carvedilol. Mitochondrion 2:305–311
Ricchelli F, Beghetto C, Gobbo S, Tognon G, Moretto V, Crisma M (2003) Structural modifications of the permeability transition pore complex in resealed mitochondria induced by matrix-entrapped disaccharides. Arch Biochem Biophys 410:155–160
Fernandes MAS, Custodio JBA, Santos MS, Moreno AJM, Vicente JAF (2006) Tetrandrine concentrations not affecting oxidative phosphorylation protect rat liver mitochondria from oxidative stress. Mitochondrion 6:176–185
Li JH, Zhang Y, Xiao Q, Tian FF, Liu XR, Li R, Zhao GY, Jiang FL, Liu Y (2011) Mitochondria as target of quantum dots toxicity. J Hazard Mater 194:440–444
Petronilli V, Šileikytė J, Zulian A, Dabbeni-Sala F, Jori G, Gobbo S, Tognon G, Nikolov P, Bernardi P, Ricchelli F (2009) Switch from inhibition to activation of the mitochondrial permeability transition during hematoporphyrin-mediated photooxidative stress. Unmasking pore-regulating external thiols BBA-Bioenergetics 1787:897–904
Zhang Y, Li JH, Liu XR, Jiang FL, Tian FF, Liu Y (2011) Spectroscopic and microscopic studies on the mechanisms of mitochondrial toxicity induced by different concentrations of cadmium. J Membrane Biol 241:39–49
Zischka H, Larochette N, Hoffmann F, Hamöller D, Jägemann N, Lichtmannegger J, Jennen L, Müller-Höcker J, Roggel F, Göttlicher M, Vollmar AM, Kroemer G (2008) Electrophoretic analysis of the mitochondrial outer membrane rupture induced by permeability transition. Anal Chem 80:5051–5058
Bodrova ME, Brailovskaya IV, Efron GI, Starkov AA, Mokhova EN (2003) Cyclosporin A-sensitive decrease in the transmembrane potential across the inner membrane of liver mitochondria induced by low concentrations of fatty acids and Ca2+. Biochemistry-Moscow 68:391–398
Malekova L, Kominkova V, Ferko M, Stefanik P, Krizanova O, Ziegelhffer A, Szewczyk A, Ondrias K (2007) Bongkrekic acid and atractyloside inhibits chloride channels from mitochondrial membranes of rat heart. BBA-Bioenergetics 1767:31–44
Liu XR, Li JH, Zhang Y, Ge YS, Tian FF, Dai J, Jiang FL, Liu Y (2011) Mitochondrial permeability transition induced by different concentrations of zinc. J Membrane Biol 244:105–112
Arpagaus S, Rawyler A, Braendle R (2002) Occurrence and characteristics of the mitochondrial permeability transition in plants. J Biol Chem 277:1780–1787
Hu X, Wang XR, Wang C (2006) Bioaccumulation of lanthanum and its effect on growth of maize seedlings in a red loamy soil. Pedosphere 16:799–805
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Acknowledgments
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (grant nos. 21077081, 21173026, and 21303126), National Science Fund for Distinguished Young Scholars of China (21225313), and Program for Changjiang Scholars and Innovative Research Team in University (IRT1030).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, J., Jin, JC., Zhou, ZQ. et al. High Concentration of Gadolinium Ion Modifying Isolated Rice Mitochondrial Biogenesis. Biol Trace Elem Res 156, 308–315 (2013). https://doi.org/10.1007/s12011-013-9821-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9821-6