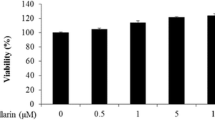
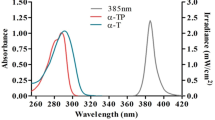
Abstract
The beneficial effect of selenium (Se) on cancer is known to depend on the chemical form, the dose and the duration of the supplementation. The aim of this work was to explore long term antagonist (antioxidant versus toxic) effects of an inorganic (sodium selenite, Na2SeO3) and an organic (seleno-L-methionine, SeMet) forms in human immortalized keratinocytes HaCaT cells. HaCaT cells were supplemented with Na2SeO3 or SeMet at micromolar concentrations for 144 h, followed or not by UVA radiation. Se absorption, effects of UVA radiation, cell morphology, antioxidant profile, cell cycle processing, DNA fragmentation, cell death triggered and caspase-3 activity were determined. At non-toxic doses (10 μM SeMet and 1 μM Na2SeO3), SeMet was better absorbed than Na2SeO3. The protection of HaCaT from UVA-induced cell death was observed only with SeMet despite both forms increased glutathione peroxidase-1 (GPX1) activities and selenoprotein-1 (SEPW1) transcript expression. After UVA irradiation, malondialdehyde (MDA) and SH groups were not modulated whatever Se chemical form. At toxic doses (100 μM SeMet and 5 μM Na2SeO3), Na2SeO3 and SeMet inhibited cell proliferation associated with S-G2 blockage and DNA fragmentation leading to apoptosis caspase-3 dependant. SeMet only led to hydrogen peroxide production and to a decrease in mitochondrial transmembrane potential. Our study of the effects of selenium on HaCaT cells reaffirm the necessity to take into account the chemical form in experimental and intervention studies.




Similar content being viewed by others
Abbreviations
- DMSO:
-
dimethylsulfoxide
- CDK:
-
cycline dependant kinase
- DCFDA:
-
2′,7′-dichlorofluorescein diacetate
- DiOC6:
-
3,3′-dihexyloxacarbocyanine iodide
- FCS:
-
foetal calf serum
- GADD:
-
growth arrest and DNA damage
- GPX1:
-
glutathione peroxidase 1
- GSR:
-
glutathione reductase
- H2Se:
-
selenide
- HBSS:
-
Hank’s buffered salt solution
- HPLC:
-
high performance liquid chromatography
- HPRT1:
-
hypoxanthine phosphoribosyltransferase 1
- ICP-MS:
-
inductively coupled plasma mass-spectrometry
- mTOR:
-
mammalian target of rapamycin
- MDA:
-
malondialdehyde
- MGG:
-
May–Grünwald Giemsa
- MI:
-
mitotic index
- MTP:
-
mitochondrial transmembrane potential
- MTT:
-
3-(4,5-dimethylthiazol- 2yl)-2,5-diphenyl tetrazolium bromide
- NHSF:
-
normal human skin fibroblast
- ROS:
-
reactive oxygen species
- SB:
-
strand break
- SeCys:
-
selenocysteine
- SeMet:
-
selenomethionine
- Na2SeO3 :
-
sodium selenite
- SEPW1:
-
selenoprotein W1
- SH:
-
thiol groups
- SOD:
-
superoxide dismutase
- SP:
-
selenoprotein
- UVA:
-
ultraviolet-A
References
Zeng H, Combs GF Jr (2008) Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem 19(1):1–7
Allan CB, Lacourciere GM, Stadtman TC (1999) Responsiveness of selenoproteins to dietary selenium. Annu Rev Nutr 19:1–16
Hesketh J (2008) Nutrigenomics and selenium: gene expression patterns, physiological targets, and genetics. Annu Rev Nutr 28:157–177
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9(7):775–806
Hatfield DL, Gladyshev VN (2002) How selenium has altered our understanding of the genetic code. Mol Cell Biol 22(11):3565–3576
Jeong D, Kim TS, Chung YW, Lee BJ, Kim IY (2002) Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Lett 517(1–3):225–228
Beilstein MA, Vendeland SC, Barofsky E, Jensen ON, Whanger PD (1996) Selenoprotein W of rat muscle binds glutathione and an unknown small molecular weight moiety. J Inorg Biochem 61(2):117–124
Hawkes WC, Alkan Z (2011) Delayed cell cycle progression from SEPW1 depletion is p53- and p21-dependent in MCF-7 breast cancer cells. Biochem Biophys Res Commun 413(1):36–40
Hawkes WC, Printsev I, Alkan Z (2009) Selenoprotein W depletion induces a p53- and p21-dependent delay in cell cycle progression in RWPE-1 prostate epithelial cells. J Cell Biochem
Hawkes WC, Printsev I, Alkan Z (2012) Selenoprotein W depletion induces a p53- and p21-dependent delay in cell cycle progression in RWPE-1 prostate epithelial cells. J Cell Biochem 113(1):61–69
Kaeck M, Lu J, Strange R, Ip C, Ganther HE, Thompson HJ (1997) Differential induction of growth arrest inducible genes by selenium compounds. Biochem Pharmacol 53(7):921–926
Zeng H (2002) Selenite and selenomethionine promote HL-60 cell cycle progression. J Nutr 132(4):674–679
Zeng H, Botnen JH, Johnson LK (2008) A selenium-deficient Caco-2 cell model for assessing differential incorporation of chemical or food selenium into glutathione peroxidase. Biol Trace Elem Res 123(1–3):98–108
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL Jr, Baker LH, Coltman CA Jr (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama 301(1):39–51
Jackson MI, Combs GF Jr (2008) Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care 11(6):718–726
Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr, Park HK, Sanders BB Jr, Smith CL, Taylor JR (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama 276(24):1957–1963
Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM Jr, Kristal AR, Santella RM, Probstfield JL, Moinpour CM, Albanes D, Taylor PR, Minasian LM, Hoque A, Thomas SM, Crowley JJ, Gaziano JM, Stanford JL, Cook ED, Fleshner NE, Lieber MM, Walther PJ, Khuri FR, Karp DD, Schwartz GG, Ford LG, Coltman CA Jr (2005) Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT). J Natl Cancer Inst 97(2):94–102
Rayman MP, Combs GF, Jr., Waters DJ (2009) Selenium and vitamin E supplementation for cancer prevention. Jama 301 (18):1876; author reply 1877
Waters DJ, Shen S, Kengeri SS, Chiang EC, Combs GF Jr, Morris JS, Bostwick DG (2012) Prostatic response to supranutritional selenium supplementation: comparison of the target tissue potency of selenomethionine vs. selenium-yeast on markers of prostatic homeostasis. Nutrients 4(11):1650–1663
Dreno B, Euvrard S, Frances C, Moyse D, Nandeuil A (2007) Effect of selenium intake on the prevention of cutaneous epithelial lesions in organ transplant recipients. Eur J Dermatol 17(2):140–145
Pence BC, Delver E, Dunn DM (1994) Effects of dietary selenium on UVB-induced skin carcinogenesis and epidermal antioxidant status. J Invest Dermatol 102(5):759–761
Burke KE, Combs GF Jr, Gross EG, Bhuyan KC, Abu-Libdeh H (1992) The effects of topical and oral L-selenomethionine on pigmentation and skin cancer induced by ultraviolet irradiation. Nutr Cancer 17(2):123–137
Leccia MT, Richard MJ, Beani JC, Faure H, Monjo AM, Cadet J, Amblard P, Favier A (1993) Protective effect of selenium and zinc on UV-A damage in human skin fibroblasts. Photochem Photobiol 58(4):548–553
Meewes C, Brenneisen P, Wenk J, Kuhr L, Ma W, Alikoski J, Poswig A, Krieg T, Scharffetter-Kochanek K (2001) Adaptive antioxidant response protects dermal fibroblasts from UVA-induced phototoxicity. Free Radic Biol Med 30(3):238–247
Rafferty TS, McKenzie RC, Hunter JA, Howie AF, Arthur JR, Nicol F, Beckett GJ (1998) Differential expression of selenoproteins by human skin cells and protection by selenium from UVB-radiation-induced cell death. Biochem J 332(Pt 1):231–236
Chen XJ, Duan FD, Zhang HH, Xiong Y, Wang J (2012) Sodium selenite-induced apoptosis mediated by ROS attack in human osteosarcoma U2OS cells. Biol Trace Elem Res 145(1):1–9
Sengupta A, Lichti UF, Carlson BA, Ryscavage AO, Gladyshev VN, Yuspa SH, Hatfield DL (2008) Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One 5(8):e12249
Park SH, Kim JH, Chi GY, Kim GY, Chang YC, Moon SK, Nam SW, Kim WJ, Yoo YH, Choi YH (2012) Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol Lett 212(3):252–261
Suzuki M, Endo M, Shinohara F, Echigo S, Rikiishi H (2011) Rapamycin suppresses ROS-dependent apoptosis caused by selenomethionine in A549 lung carcinoma cells. Cancer Chemother Pharmacol 67(5):1129–1136
Sanmartin C, Plano D, Sharma AK, Palop JA (2012) Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. Int J Mol Sci 13(8):9649–9672
Emonet-Piccardi N, Richard MJ, Ravanat JL, Signorini N, Cadet J, Beani JC (1998) Protective effects of antioxidants against UVA-induced DNA damage in human skin fibroblasts in culture. Free Radic Res 29(4):307–313
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Champelovier P, Mininno M, Duchamp E, Nicolle E, Curri V, Boumendjel A, Boutonnat J (2011) Cytotoxicity of chalcone derivatives towards glioblastoma. Anticancer Res 31(10):3213–3218
Ozgen U, Savasan S, Buck S, Ravindranath Y (2000) Comparison of DiOC(6)(3) uptake and annexin V labeling for quantification of apoptosis in leukemia cells and non-malignant T lymphocytes from children. Cytometry 42(1):74–78
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C (2001) An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25(4):386–401
Mukai FH, Goldstein BD (1976) Mutagenicity of malonaldehyde, a decomposition product of peroxidized polyunsaturated fatty acids. Science 191(4229):868–869
Richard MJ, Guiraud P, Meo J, Favier A (1992) High-performance liquid chromatographic separation of malondialdehyde-thiobarbituric acid adduct in biological materials (plasma and human cells) using a commercially available reagent. J Chromatogr 577(1):9–18
Bulaj G, Kortemme T, Goldenberg DP (1998) Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 37(25):8965–8972
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Boukamp P (2005) UV-induced skin cancer: similarities–variations. J Dtsch Dermatol Ges 3(7):493–503
Lunoe K, Gabel-Jensen C, Sturup S, Andresen L, Skov S, Gammelgaard B (2011) Investigation of the selenium metabolism in cancer cell lines. Metallomics 3(2):162–168
Gammelgaard B, Rasmussen LH, Gabel-Jensen C, Steffansen B (2012) Estimating Intestinal Absorption of Inorganic and Organic Selenium Compounds by in Vitro Flux and Biotransformation Studies in Caco-2 Cells and ICP-MS Detection. Biol Trace Elem Res 145(2):248–256
Leblondel G, Mauras Y, Cailleux A, Allain P (2001) Transport measurements across Caco-2 monolayers of different organic and inorganic selenium: influence of sulfur compounds. Biol Trace Elem Res 83(3):191–206
Finley JW (2005) Selenium accumulation in plant foods. Nutr Rev 63(6 Pt 1):196–202
Thomson CD, Robinson MF, Butler JA, Whanger PD (1993) Long-term supplementation with selenate and selenomethionine: selenium and glutathione peroxidase (EC 1.11.1.9) in blood components of New Zealand women. Br J Nutr 69(2):577–588
Wastney ME, Combs GF Jr, Canfield WK, Taylor PR, Patterson KY, Hill AD, Moler JE, Patterson BH (2011) A human model of selenium that integrates metabolism from selenite and selenomethionine. J Nutr 141(4):708–717
Whanger PD (2002) Selenocompounds in plants and animals and their biological significance. J Am Coll Nutr 21(3):223–232
Caffrey PB, Frenkel GD (1997) Sensitivity of melphalan-resistant tumors to selenite in vivo. Cancer Lett 121(2):177–180
Zuo L, Li J, Yang Y, Wang X, Shen T, Xu CM, Zhang ZN (2004) Sodium selenite induces apoptosis in acute promyelocytic leukemia-derived NB4 cells by a caspase-3-dependent mechanism and a redox pathway different from that of arsenic trioxide. Ann Hematol 83(12):751–758
McKeehan WL, Hamilton WG, Ham RG (1976) Selenium is an essential trace nutrient for growth of WI-38 diploid human fibroblasts. Proc Natl Acad Sci U S A 73(6):2023–2027
Lu J, Jiang C, Kaeck M, Ganther H, Ip C, Thompson H (1995) Cellular and metabolic effects of triphenylselenonium chloride in a mammary cell culture model. Carcinogenesis 16(3):513–517
Sinha R, Said TK, Medina D (1996) Organic and inorganic selenium compounds inhibit mouse mammary cell growth in vitro by different cellular pathways. Cancer Lett 107(2):277–284
Thompson HJ, Wilson A, Lu J, Singh M, Jiang C, Upadhyaya P, el-Bayoumy K, Ip C (1994) Comparison of the effects of an organic and an inorganic form of selenium on a mammary carcinoma cell line. Carcinogenesis 15(2):183–186
Kim TS, Yun BY, Kim IY (2003) Induction of the mitochondrial permeability transition by selenium compounds mediated by oxidation of the protein thiol groups and generation of the superoxide. Biochem Pharmacol 66(12):2301–2311
Zeng H, Briske-Anderson M, Idso JP, Hunt CD (2006) The selenium metabolite methylselenol inhibits the migration and invasion potential of HT1080 tumor cells. J Nutr 136(6):1528–1532
Zeng H, Wu M, Botnen JH (2009) Methylselenol, a selenium metabolite, induces cell cycle arrest in G1 phase and apoptosis via the extracellular-regulated kinase 1/2 pathway and other cancer signaling genes. J Nutr 139(9):1613–1618
Jin RJ, Lho Y, Wang Y, Ao M, Revelo MP, Hayward SW, Wills ML, Logan SK, Zhang P, Matusik RJ (2008) Down-regulation of p57Kip2 induces prostate cancer in the mouse. Cancer Res 68(10):3601–3608
Ganther HE (1999) Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis 20(9):1657–1666
Ip C (1998) Lessons from basic research in selenium and cancer prevention. J Nutr 128(11):1845–1854
Esaki N, Tanaka H, Uemura S, Suzuki T, Soda K (1979) Catalytic action of L-methionine gamma-lyase on selenomethionine and selenols. Biochemistry 18(3):407–410
Kim EH, Sohn S, Kwon HJ, Kim SU, Kim MJ, Lee SJ, Choi KS (2007) Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res 67(13):6314–6324
Yoon SO, Kim MM, Chung AS (2001) Inhibitory effect of selenite on invasion of HT1080 tumor cells. J Biol Chem 276(23):20085–20092
Yoon SO, Kim MM, Park SJ, Kim D, Chung J, Chung AS (2002) Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J 16(1):111–113
Snyder RD (1987) Effects of sodium selenite on DNA and carcinogen-induced DNA repair in human diploid fibroblasts. Cancer Lett 34(1):73–81
Kralova V, Benesova S, Cervinka M, Rudolf E (2012) Selenite-induced apoptosis and autophagy in colon cancer cells. Toxicol In Vitro 26(2):258–268
Yang Y, Huang F, Ren Y, Xing L, Wu Y, Li Z, Pan H, Xu C (2009) The anticancer effects of sodium selenite and selenomethionine on human colorectal carcinoma cell lines in nude mice. Oncol Res 18(1):1–8
Bjornstedt M, Kumar S, Bjorkhem L, Spyrou G, Holmgren A (1997) Selenium and the thioredoxin and glutaredoxin systems. Biomed Environ Sci 10(2–3):271–279
Acknowledgments
We would like to thank all the technicians for their technical assistance: Aurélie Dariz and Michelle Tripier-Champ for cell cultures, toxicity assays and RT-q-PCR, Sandra Grange and Angèle Kraviec for GPX1 activities and SH-groups determination, Laurence Puillet for MDA measurements, Marie-Christine Bouillet and Dominique André for selenium measurements.
Declaration of Interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hazane-Puch, F., Champelovier, P., Arnaud, J. et al. Long-Term Selenium Supplementation in HaCaT Cells: Importance of Chemical Form for Antagonist (Protective Versus Toxic) Activities. Biol Trace Elem Res 154, 288–298 (2013). https://doi.org/10.1007/s12011-013-9709-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9709-5