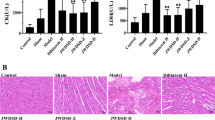
Abstract
Intestinal ischemia–reperfusion (II/R) injury is a complex pathologic process, which is of great significance to unravel the underlying mechanisms and pathophysiology. Our study represented a comprehensive proteomic analysis in the human intestine with ischemia–reperfusion injury. The proteomics analysis measured a total of 5,230 proteins, and 417 differently expressed proteins (DEPs) were identified between II/R and control samples. GO and KEGG analysis demonstrated that the 290 upregulated DEPs in II/R were significantly involved in immune-related biological process and tight junction, focal adhesion, and cAMP signaling pathway, whereas the 127 downregulated DEPs in II/R were enriched in lipid metabolic process and metabolic pathway. Furthermore, we screened out 20 hub proteins from the protein–protein interaction (PPI) network according to the degree of connectivity, and six clusters were identified. Combined with the result of KEGG analysis, 6 from the 20 hub proteins, ACTB, CAV1, FLNA, MYLK, ACTN1, and MYL9, were identified as the key proteins in the progress of II/R injury. According to the previous studies, FLNA and MYL9 were selected as the novel disease-related proteins for the first time. In conclusion, this study extended our understanding of the alteration in the human intestine during ischemia and reperfusion and highlighted the potential role of FLNA and MYL9 in the progress of II/R injury, which need to be further studied.





Similar content being viewed by others
Data Availability
The mass spectrometry proteomics data have been deposited to the Proteome Xchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD033218.
Abbreviations
- DDA:
-
Data-dependent acquisition
- DIA:
-
Data-independent acquisition
- II/R:
-
Intestinal ischemia–reperfusion
- LC–MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- DEP:
-
Differently expressed proteins
- PPI:
-
Protein–protein interaction
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GO:
-
Gene Ontology
- ROS:
-
Reactive oxygen species
- ICU:
-
Intensive care unit
- PCA:
-
Principal component analysis
- STRING:
-
Search Tool for the Retrieval of Interacting Genes
- MCODE:
-
Molecular Complex Detection
- LFQ:
-
Label-free quantification
References
Jiang, Z., Chen, S., Zhang, L., Shen, J., & Zhong, M. (2021). Potentially functional microRNA-mRNA regulatory networks in intestinal ischemia-reperfusion injury: A bioinformatics analysis. Journal of Inflammation Research, 14, 4817–4825.
Mazzei, M. A. (2018). Acute mesenteric ischemia: Guidelines of the World Society of Emergency Surgery: A brief radiological commentary. World J Emerg Surgery, 13, 34.
Oldenburg, W. A., Lau, L. L., Rodenberg, T. J., Edmonds, H. J., & Burger, C. D. (2004). Acute mesenteric ischemia: A clinical review. Archives of Internal Medicine, 164, 1054–1062.
Malinovic, M., Walker, J., & Lee, F. (2021). Ischemia-reperfusion injury after posterior cervical laminectomy. Cureus, 13, e18298.
Grootjans, J., Lenaerts, K., Derikx, J. P., Matthijsen, R. A., de Bruïne, A. P., van Bijnen, A. A., van Dam, R. M., Dejong, C. H., & Buurman, W. A. (2010). Human intestinal ischemia-reperfusion-induced inflammation characterized: Experiences from a new translational model. American Journal of Pathology, 176, 2283–2291.
Grootjans, J., Thuijls, G., Derikx, J. P., van Dam, R. M., Dejong, C. H., & Buurman, W. A. (2011). Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia–reperfusion. The Journal of Pathology, 224, 411–419.
Grootjans, J., Hodin, C. M., de Haan, J. J., Derikx, J. P., Rouschop, K. M., Verheyen, F. K., van Dam, R. M., Dejong, C. H., Buurman, W. A., & Lenaerts, K. (2011). Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology, 140, 529-539.e523.
Mastoraki, A., Mastoraki, S., Tziava, E., Touloumi, S., Krinos, N., Danias, N., Lazaris, A., & Arkadopoulos, N. (2016). Mesenteric ischemia: Pathogenesis and challenging diagnostic and therapeutic modalities. World J Gastrointest Pathophysiol, 7, 125–130.
Fahrner, M., Föll, M. C., Grüning, B. A., Bernt, M., Röst, H. and Schilling, O. (2022) Democratizing data-independent acquisition proteomics analysis on public cloud infrastructures via the Galaxy framework. Gigascience, 11.
Li, Y. S., Wang, Z. X., Li, C., Xu, M., Li, Y., Huang, W. Q., Xia, Z., & Liu, K. X. (2010). Proteomics of ischemia/reperfusion injury in rat intestine with and without ischemic postconditioning. Journal of Surgical Research, 164, e173-180.
Liu, K. X., Li, C., Li, Y. S., Yuan, B. L., Xu, M., Xia, Z., & Huang, W. Q. (2010). Proteomic analysis of intestinal ischemia/reperfusion injury and ischemic preconditioning in rats reveals the protective role of aldose reductase. Proteomics, 10, 4463–4475.
Wen, S. H., Ling, Y. H., Li, Y., Li, C., Liu, J. X., Li, Y. S., Yao, X., Xia, Z. Q., & Liu, K. X. (2013). Ischemic postconditioning during reperfusion attenuates oxidative stress and intestinal mucosal apoptosis induced by intestinal ischemia/reperfusion via aldose reductase. Surgery, 153, 555–564.
Kip, A. M., Valverde, J. M., Altelaar, M., Heeren, R. M. A., Hundscheid, I. H. R., Dejong, C. H. C., Olde Damink, S. W. M., Balluff, B., & Lenaerts, K. (2022). Combined quantitative (phospho)proteomics and mass spectrometry imaging reveal temporal and spatial protein changes in human intestinal ischemia-reperfusion. Journal of Proteome Research, 21, 49–66.
Kip, A. M., Soons, Z., Mohren, R., Duivenvoorden, A. A. M., Röth, A. A. J., Cillero-Pastor, B., Neumann, U. P., Dejong, C. H. C., Heeren, R. M. A., Olde Damink, S. W. M., & Lenaerts, K. (2021). Proteomics analysis of human intestinal organoids during hypoxia and reoxygenation as a model to study ischemia-reperfusion injury. Cell Death & Disease, 12, 95.
Wang, B., Qu, X. L., & Chen, Y. (2019). Identification of the potential prognostic genes of human melanoma. Journal of Cellular Physiology, 234, 9810–9815.
Xie, F., Wang, M., Su, Y., Xiao, K., Chu, X., Long, S., Li, L., Zhang, X., Xue, P., & Zhu, S. (2022). Unveiling potential mechanisms of Spatholobi Caulis against lung metastasis of malignant tumor by network pharmacology and molecular docking. Evid Based Complement Alternat Med, 2022, 1620539.
Lu, X., Li, Y., Simovic, M. O., Peckham, R., Wang, Y., Tsokos, G. C., & Dalle Lucca, J. J. (2011). Decay-accelerating factor attenuates C-reactive protein-potentiated tissue injury after mesenteric ischemia/reperfusion. Journal of Surgical Research, 167, e103-115.
Rehm, S. R. T., Smirnova, N. F., Morrone, C., Götzfried, J., Feuchtinger, A., Pedersen, J., Korkmaz, B., Yildirim, A., & Jenne, D. E. (2019). Premedication with a cathepsin C inhibitor alleviates early primary graft dysfunction in mouse recipients after lung transplantation. Science and Reports, 9, 9925.
Fang, H., Liu, A., Dirsch, O., & Dahmen, U. (2013). Liver transplantation and inflammation: Is lipopolysaccharide binding protein the link? Cytokine, 64, 71–78.
Kong, J., Zhang, Z. Y., Musch, M. W., Ning, G., Sun, J., Hart, J., Bissonnette, M., & Li, Y. C. (2008). Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol-Gastr L, 294, G208–G216.
Gu, L. L., Li, N., Yu, W. K., Gong, J. F., Li, Q. R., Zhu, W. M., & Li, J. S. (2013). Berberine reduces rat intestinal tight junction injury induced by ischemia-reperfusion associated with the suppression of inducible nitric oxide synthesis. Am J Chinese Med, 41, 1297–1312.
Watson, A. J. M., Chu, S. Y., Sieck, L., Gerasimenko, O., Bullen, T., Campbell, F., McKenna, M., Rose, T., & Montrose, M. H. (2005). Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology, 129, 902–912.
Wu, C., Wang, X. Y., Jiang, T. T., Li, C. J., Zhang, L., Gao, X. J., Tian, F., Li, N. and Li, J. S. (2016) Partial enteral nutrition mitigated ischemia/reperfusion-induced damage of rat small intestinal barrier. Nutrients, 8.
Suzuki, T. (2020) Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J, 91.
Evennett, N., Cerigioni, E., Hall, N. J., Pierro, A., & Eaton, S. (2014). Smooth muscle actin as a novel serologic marker of severe intestinal damage in rat intestinal ischemia-reperfusion and human necrotising enterocolitis. Journal of Surgical Research, 191, 323–330.
Shi, T., Moulton, V. R., Lapchak, P. H., Deng, G. M., Dalle Lucca, J. J., & Tsokos, G. C. (2009). Ischemia-mediated aggregation of the actin cytoskeleton is one of the major initial events resulting in ischemia-reperfusion injury. American Journal of Physiology. Gastrointestinal and Liver Physiology, 296, G339-347.
Deng, F., Zhang, L.-Q., Wu, H., Chen, Y., Yu, W.-Q., Han, R.-H., Han, Y., Zhang, X.-Q., Sun, Q.-S., Lin, Z.-B., Wang, Y., Liu, Y.-P., Chen, J.-Y., Liu, K.-X., & Hu, J.-J. (2022). Propionate alleviates myocardial ischemia-reperfusion injury aggravated by angiotensin II dependent on caveolin-1/ACE2 axis through GPR41. International Journal of Biological Sciences, 18, 858–872.
Jin, Y., & Blikslager, A. T. (2016). Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury. American Journal of Physiology. Cell Physiology, 311, C996-c1004.
Karimi, A., & Milewicz, D. M. (2016). Structure of the elastin-contractile units in the thoracic aorta and how genes that cause thoracic aortic aneurysms and dissections disrupt this structure. Canadian Journal of Cardiology, 32, 26–34.
Yokoyama, M., Kimura, M. Y., Ito, T., Hayashizaki, K., Endo, Y., Wang, Y., Yagi, R., Nakagawa, T., Kato, N., Matsubara, H., & Nakayama, T. (2020). Myosin light chain 9/12 regulates the pathogenesis of inflammatory bowel disease. Frontiers in Immunology, 11, 594297.
Liu, S. Z., He, X. M., Zhang, X., Zeng, F. C., Wang, F., & Zhou, X. Y. (2017). Ischemic preconditioning-induced SOCS-1 protects rat intestinal ischemia reperfusion injury via degradation of TRAF6. Digestive Diseases and Sciences, 62, 105–114.
Li, Y., Feng, D., Wang, Z., Zhao, Y., Sun, R., Tian, D., Liu, D., Zhang, F., Ning, S., Yao, J., & Tian, X. (2019). Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death and Differentiation, 26, 2284–2299.
Mabrouk, N., Lecoeur, B., Bettaieb, A., Paul, C. and Vegran, F. (2022) Impact of lipid metabolism on antitumor immune response. Cancers, 14.
Castoldi, A., Monteiro, L. B., Bakker, N. V., Sanin, D. E., Rana, N., Corrado, M., Cameron, A. M., Hassler, F., Matsushita, M., Caputa, G., Geltink, R. I. K., Buscher, J., Edwards-Hicks, J., Pearce, E. L. and Pearce, E. J. (2020) Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun, 11.
Hung, Y. H., Carreiro, A. L., & Buhman, K. K. (2017). Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids, 1862, 600–614.
Funken, D., Yu, Y., Feng, X., Imvised, T., Gueler, F., Prinz, I., Madadi-Sanjani, O., Ure, B. M., Kuebler, J. F., & Klemann, C. (2021). Lack of gamma delta T cells ameliorates inflammatory response after acute intestinal ischemia reperfusion in mice. Science and Reports, 11, 18628.
Campos, M. A., Rosinha, G. M., Almeida, I. C., Salgueiro, X. S., Jarvis, B. W., Splitter, G. A., Qureshi, N., Bruna-Romero, O., Gazzinelli, R. T., & Oliveira, S. C. (2004). Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infection and Immunity, 72, 176–186.
Aldrich, M. B., & Sevick-Muraca, E. M. (2013). Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine, 64, 362–369.
Wu, H., Deng, Y. Y., Liu, L., Tan, Q. H., Wang, C. H., Guo, M. M., Xie, Y. M., & Tang, C. W. (2014). Intestinal ischemia-reperfusion of macaques triggers a strong innate immune response. World Journal of Gastroenterology, 20, 15327–15334.
Kodiha, M., Chu, A., Matusiewicz, N., & Stochaj, U. (2004). Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death & Differentiation, 11, 862–874.
Schwartz, M. A., & DeSimone, D. W. (2008). Cell adhesion receptors in mechanotransduction. Current Opinion in Cell Biology, 20, 551–556.
Petit, V., & Thiery, J. P. (2000). Focal adhesions: Structure and dynamics. Biology of the Cell, 92, 477–494.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81801943), the Science and Technology Commission of Shanghai Municipality (No.18411970200), the Shanghai Pujiang Program (No. 21PJD009), and the Medical and Health Science and Technology Innovation Fund Project in Jinshan District, Shanghai (No.2020–3-19).
Author information
Authors and Affiliations
Contributions
JBQ, MZ, and LZ conceived and designed the research; AZH collected the data used in our study; AZH and WW analyzed the data; AZH, SC, and HBH draw the figures; WW and JS wrote the manuscript; and MZ and LZ approved the final version of manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Shanghai Medical College of Fudan University.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, A., Wu, W., Chen, S. et al. Data-Independent Acquisition-Based Mass Spectrometry (DIA-MS) for Quantitative Analysis of Human Intestinal Ischemia/Reperfusion. Appl Biochem Biotechnol 194, 4156–4168 (2022). https://doi.org/10.1007/s12010-022-04005-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04005-4