Abstract
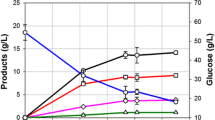
Kariba weed (Salvinia molesta) was used as biomass feedstock for ethanol production by separate hydrolysis and fermentation (SHF). Monosaccharides from Kariba weed hydrolysate were produced using thermal acid hydrolysis, sonication, and enzymatic saccharification. The optimal conditions for thermal acid hydrolysis of 12% (w/v) Kariba weed slurry were evaluated as 200 mM HNO3 at 121 °C for 60 min yielding 10.2 g/L monosaccharides. Sonication for 45 min before enzymatic saccharification yielded more monosaccharides to 18.7 g/L. Enzymatic saccharification with 16 U/mL Cellic CTec2 produced 35.4 g/L monosaccharides. Fermentation was performed using Saccharomyces cerevisiae, Kluyveromyces marxianus, or Pichia stipitis with sonicated Kariba weed hydrolysate. The control fermentations were carried out using Kariba weed hydrolysate without sonication. The improvement of ethanol production from sonicated Kariba weed hydrolysate using P. stipitis produced 15.9 g/L ethanol with ethanol yield coefficient YEtOH = 0.45, K. marxianus produced 14.7 g/L ethanol with YEtOH = 0.41. S. cerevisiae produced the lowest yield of 13.2 g/L ethanol with YEtOH = 0.37 as it utilized only glucose not xylose. Sonication of Kariba weed was essential in the ethanol production to enhance the productivity of monosaccharides. P. stipitis was determined as the best yeast species using hydrolysates with the mixture of glucose and xylose to produce ethanol.







Similar content being viewed by others
References
Chiari, L., & Zecca, A. (2011). Constraints of fossil fuels depletion on global warming projections. Energy Policy, 39(9), 5026–5034.
Schenk, P. M., & Thomas-hall, S. R. (2008). Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Research, 1, 20–43.
Dias, M. O. S., Ensinas, A. V., Nebra, S. A., Maciel, R., Rossell, C. E. V., Regina, M., & Maciel, W. (2009). Production of bioethanol and other bio-based materials from sugarcane bagasse: integration to conventional bioethanol production process. Chemical Engineering Research and Design, 87(9), 1206–1216.
Khan, S. (2009). Prospects of biodiesel production from microalgae in India. Renewable and Sustainable Energy Reviews, 13(2009), 2361–2372.
Chiaramonti, D., Prussi, M., Ferrero, S., Oriani, L., Ottonello, P., Torre, P., & Cherchi, F. (2012). Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass and Bioenergy, 46, 25–35.
Alvira, P., Tomás-Pejó, E., Ballesteros, M., & Negro, M. J. (2010). Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresource Technology, 101(13), 4851–4861.
Ghadiryanfar, M., Rosentrater, K. A., Keyhani, A., & Omid, M. (2016). A review of macroalgae production, with potential applications in biofuels and bioenergy. Renewable and Sustainable Energy Reviews, 54, 473–481.
Mcintosh, D., King, C., & Fitzsimmons, K. (2003). Tilapia for biological control of giant salvinia. Journal of Aquatic Plant Management, 41, 28–31.
Witt, A., Witt, A., Beale, T., & Van Wilgen, B. W. (2018). An assessment of the distribution and potential ecological impacts of invasive alien plant species in eastern Africa. Transactions of the Royal Society of South Africa, 73(3), 217–236.
Malik, A. (2007). Environmental challenge vis a vis opportunity: the case of water hyacinth. Environment International, 33(1), 122–138.
Andama, M., Ongom, R., & Lukubye, B. (2017). Proliferation of Salvinia molesta at lake Kyoga landing sites as a result of anthropogenic influences. Journal of Geoscience and Environment Protection., 5, 160–173.
Bruszt, G., Ammour, T., Claussen, J., Ofir, Z., Saxena, N. C., & Turner, S. (2003). IUCN – The world conservation union external review. Gland: IUCN – The World Conservation Union.
Owens, C., & Dick, G. O. (2015). Effects of pH on growth of Salvinia molesta Mitchell. Journal of Aquatic Plant Management, 43, 34–38.
Bamulangaki Sempijja, V. (2019). Allocate funds control aquatic weed kadaga. Minister of agriculture animal industry and fisheries, Minister of Agriculture, Uganda.
Kaur, M., Kumar, M., Sachdeva, S., & Puri, S. K. (2018). Aquatic weeds as the next generation feedstock for sustainable bioenergy production. Bioresource Technology, 251, 390–402.
Namadi, M. M. (2013). Evaluation of sugar content and bioethanol potentials of some freshwater biomass. Journal of Renewable and Sustainable Energy, 2(6), 201–204.
Mubarak, M., Gupta, P., Shaija, A., & Suchithra, T. V. (2017). Production of bioethanol from Salvinia molesta and its utilization in single cylinder SI engine. Journal of Advance in Engineering Research, 4(1), 67–72.
Thomas Klasson, K. (2009). Acceleration of the enzymatic hydrolysis of corn stover and sugar cane bagasse celluloses by low intensity uniform ultrasound. Journal of Biobased Materials and Bioenergy, 3(1), 25–z31.
AOAC (Association of Official Analysis Chemists). (1995). Official methods of analysis of the association of official analytical chemists. Arlington: Association of Official Analysis Chemists.
Moozhiyil, M., & Pallauf, J. (1986). Chemical composition of the water fern, Salvinia molesta, and its potential as feed source for ruminants. Economic Botany, 40(3), 375–383.
Zouhair, F. Z., Benali, A., Kabbour, M. R., EL Kabous, K., El Maadoudi, E. h., Bouksaim, M., & Essamri, A. (2018). Typical characterization of argane pulp of various Moroccan areas: a new biomass for the second generation bioethanol production. Journal of the Saudi Society of Agricultural SciencesIn press. https://doi.org/10.1016/j.jssas.2018.09.004.
Room, P. M., & Thomas, P. A. (1986). Nitrogen, phosphorus and potassium in Salvinia molesta Mitchell in the field: effects of weather, insect damage, fertilizers and age. Aquatic Botany. https://doi.org/10.1016/0304-3770(86)90058-6.
Lu, X., Zhang, Y., & Angelidaki, I. (2009). Optimization of H2SO4-catalyzed hydrothermal pretreatment of rapeseed straw for bioconversion to ethanol: focusing on pretreatment at high solids content. Bioresource Technology, 100(12), 3048–3053.
Rosgaard, L., Andric, P., Dam-Johansen, K., Pedersen, S., & Meyer, A. S. (2007). Effects of substrate loading on enzymatic hydrolysis and viscosity of pretreated barley straw. Applied Biochemistry and Biotechnology, 143(1), 27–40.
Redding, A. P., Wang, Z., Keshwani, D. R., Cheng, J. J., Redding, A. P., Wang, Z., & Keshwani, D. R. (2010). High temperature dilute acid pretreatment of coastal Bermuda grass for enzymatic hydrolysis. Bioresource Technology, 102(2), 1415–1424.
Sukwong, P., Sunwoo, I. Y., Jeong, D. Y., Kim, S. R., Jeong, G. T., & Kim, S. K. (2019). Improvement of bioethanol production by Saccharomyces cerevisiae through the deletion of GLK1, MIG1 and MIG2 and overexpression of PGM2 using the red seaweed Gracilaria verrucosa. Process Biochemistry, 89, 134–145.
Dziekońska-Kubczak, U., Berłowska, J., Dziugan, P., Patelski, P., Pielech-Przybylska, K., & Balcerek, M. (2018). Nitric acid pretreatment of Jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies, 11(8), 2153–2169.
Badal, C. S., Loren, B. I., Michael, A. C., & Victor, Y. W. (2005). Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Biotechnology Progress, 21(3), 816–822.
Ur Rehman, M. S., Kim, I., Chisti, Y., & Han, J. I. (2012). Use of ultrasound in the production of bioethanol from lignocellulosic biomass. Energy Education Science and Technology Part A: Energy Science and Research, 30(2), 1931–1410.
Luo, J., Fang, Z., & Smith, R. L. (2014). Ultrasound-enhanced conversion of biomass to biofuels. Progress in Energy and Combustion Science, 41(1), 56–93.
Hahn-Hägerdal, B., Galbe, M., Gorwa-Grauslund, M. F., Lidén, G., & Zacchi, G. (2006). Bio-ethanol - the fuel of tomorrow from the residues of today. Trends in Biotechnology, 24(12), 549–556.
Sunwoo, I. Y., Nguyen, T. H., Sukwong, P., Jeong, G. T., & Kim, S. K. (2018). Enhancement of ethanol production via hyper thermal acid hydrolysis and co-fermentation using waste seaweed from Gwangalli Beach, Busan. Korea. J. Microbiol. Biotechnol., 28(3), 401–408.
Singla, A., Paroda, S., Dhamija, S. S., Goyal, S., Shekhawat, K., Amachi, S., & Inubushi, K. (2012). Bioethanol production from xylose: problems and possibilities. Journal of Biofuels, 3(1), 39–49.
Ofori-Boateng, C., & Lee, K. T. (2014). Ultrasonic-assisted simultaneous saccharification and fermentation of pretreated oil palm fronds for sustainable bioethanol production. Fuel, 119, 285–291.
SuperPro Designer ® Intelligen Suit TM, 908, 74–102. Available from www.Kuhnunsa.co.kr
Rogers, P. L., Jeon, Y. J., & Svenson, C. J. (2006). Application of biotechnology to industrial sustainability. Process. Saf. Environ., 84(5), 329–336.
Bussemaker, M. J., & Zhang, D. (2013, March 13). Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Industrial and Engineering Chemistry Research, 52(10), 3563–3580.
Katahira, S., Mizuike, A., Fukuda, H., & Kondo, A. (2006). Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain. Applied Microbiology and Biotechnology, 72(6), 1136–1143.
Kim, S. K., Kong, I. S., Kong, J. Y., Kim, Y. S., & Park, D. H. (1995). Process simulation for the production of porcine growth hormone using CAD program. KSBB J., 10(1), 97–104.
Gandla, M. L., Martin, C., & Leif, J. J. (2018). Analytical enzymatic saccharification of lignocellulosic biomass for conversion to biofuels and bio-based chemicals. Energies, 11(11), 2936–2955.
Ghadiryanfar, M., Rosentrater, K. A., Keyhani, A., & Omid, M. (2015). A review of macroalgae production, with potential applications in biofuels and bioenergy. Renewable and Sustainable Energy Reviews, 96(54), 473–481.
Ganguly, A., Chatterjee, P. K., & Dey, A. (2012). Studies on ethanol production from water hyacinth - a review. Renewable and Sustainable Energy Reviews, 16(1), 966–972.
Balat, M., Balat, H., & Öz, C. (2008). Progress in bioethanol processing. Prog. Energ. Combust., 34(5), 551–573.
Eggeman, T., & Elander, R. T. (2005). Process and economic analysis of pretreatment technologies. Bioresource Technology, 96(18), 2019–2025.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Moses Katongole Kityo and Inyung Sunwoo are co-first author
Rights and permissions
About this article
Cite this article
Kityo, M.K., Sunwoo, I., Kim, S.H. et al. Enhanced Bioethanol Fermentation by Sonication Using Three Yeasts Species and Kariba Weed (Salvinia molesta) as Biomass Collected from Lake Victoria, Uganda. Appl Biochem Biotechnol 192, 180–195 (2020). https://doi.org/10.1007/s12010-020-03305-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03305-x