Abstract
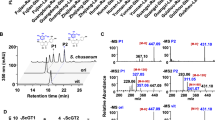
Flavonoids have gained much attention for their proposed positive effects for human health. Glycosylation is a significant method for the structural modification of various flavanols, resulting in glycosides with increased solubility, stability, and bioavailability compared with the corresponding aglycone. Natural product glycosylation by using enzymes has emerged as a topic of interest as it offers a sustainable and economical alternative source so as to address supply scalability limitations associated with plant-based production. Quercetin-3,4′-O-diglucoside, as one of the major but trace bioactive flavonoids in onion (Allium cepa), is superior or at least equal to quercetin aglycone in its bioavailability. In the present study, the onion-derived enzyme, UGT73G1, coupled with sucrose synthase, StSUS1, from Solanum tuberosum formed a circulatory system to produce quercetin-3,4′-O-diglucoside from quercetin, which preferred sucrose as a sugar donor and quercetin as a sugar acceptor. The optimal conditions were determined in order to increase the production of quercetin-3,4′-O-diglucoside. The maximum concentration of quercetin-3,4′-O-diglucoside achieved in a 10-mL reaction was 427.11 mg/L, from the conversion of 1 g/L of quercetin for 16 h at 40 °C and pH 7.2.




Similar content being viewed by others
References
Dixon, R. A., & Steele, C. L. (1999). Flavonoids and isoflavonoids-a gold mine for metabolic engineering. Trends in Plant Science, 4(10), 394–400.
Halliwell, B., Rafter, J., & Jenner, A. (2005). Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? The American Journal of Clinical Nutrition, 81(1), 268S–276S.
Nicolle, E., Souard, F., Faure, P., & Boumendjel, A. (2011). Flavonoids as promising lead compounds in type 2 diabetes mellitus: molecules of interest and structure–activity relationship. Current Medicinal Chemistry, 18(17), 2661–2672.
Jeong, J., An, J. Y., Kwon, Y. T., Rhee, J. G., & Lee, Y. J. (2009). Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. Journal of Cellular Biochemistry, 106(1), 73–82.
Luo, H., Jiang, B., King, S. M., & Chen, Y. C. (2008). Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutrition and Cancer, 60(6), 800–809.
Choi, E. J., Bae, S. M., & Ahn, W. S. (2008). Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Archives of Pharmacal Research, 31(10), 1281–1285.
Zielinska, M., Gülden, M., & Seibert, H. (2003). Effects of quercetin and quercetin-3-O-glycosides on oxidative damage in rat C6 glioma cells. Environmental Toxicology and Chemistry, 13, 47–53.
Kim, Y., Narayanan, S., & Chang, K. O. (2010). Inhibition of influenza virus replication by plant-derived isoquercitrin. Antiviral Research, 88(2), 227–235.
Liang, D., Liu, J., Wu, H., Wang, B., Zhu, H., & Qiao, J. (2015). Glycosyltransferases: mechanisms and applications in natural product development. Chemical Society Reviews, 44(22), 8350–8374.
Ramos, S. (2007). Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. The Journal of Nutritional Biochemistry, 18(7), 427–442.
Makino, T., Kanemaru, M., Okuyama, S., Shimizu, R., Tanaka, H., & Mizukami, H. (2013). Anti-allergic effects of enzymatically modified isoquercitrin (α-oligoglucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglucosyl rutin, and quercetin, when administered orally to mice. Chinese Journal of Natural Medicines, 61, 881–886.
Nile, S. H., Nile, A. S., Keum, Y. S., & Sharma, K. (2017). Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chemistry, 235, 119–126.
Kashion, Y., Murota, K., Matsuda, N., Tomotake, M., Hamano, T., Mukai, R., & Terao, J. (2015). Effect of processed onions on the plasma concentration of quercetin in rats and humans. Journal of Food Science, 80(11), H2597–H2602.
Beesk, N., Perner, H. S., Dietmar, G., Eckhard, K., Lothar, W., & Sascha, R. (2010). Distribution of quercetin-3,4’-O-diglucoside, quercetin-4’-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chemistry, 122(3), 566–571.
Kramer, C. M., Prata, R. T., Willits, M. G., Luca, D. V., Steffens, J. C., & Graser, G. (2003). Cloning and regiospecificity studies of two flavonoid glucosyltransferases from Allium cepa. Phytochemistry, 64(6), 1069–1076.
Xiao, J. B., Muzashvili, T. S., & Georgiev, M. I. (2014). Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnology Advances, 32(6), 1145–1156.
Schmölzer, K., Gutmann, A., Diricks, M., Desmet, T., & Nidetzky, B. (2016). Sucrose synthase: a unique glycosyltransferase for biocatalytic glycosylation process development. Biotechnology Advances, 34(2), 88–111.
Elling, L., Rupprath, C., Gunther, N., Römer, U., Verseck, S., Weingarten, P., Gerald, D., & Andreas, K. (2005). An enzyme module system for the synthesis of dTDP-activated deoxysugars from dTMP and sucrose. Chem Bio Chem, 6(8), 1423–1430.
Masada, S., Kawase, Y., Nagatoshi, M., Qguchi, Y., Terasaka, K., & Mizukami, H. (2007). An efficient chemoenzymatic production of small molecule glucosides with in situ UDP-glucose recycling. FEBS Letters, 581(13), 2562–2566.
Bungaruang, L., Gutmann, A., & Nidetzky, B. (2013). Leloir glycosyltransferases and natural product glycosylation: biocatalytic synthesis of the C-glucoside nothofagin, a major antioxidant of red bush herbal tea. Advanced Synthesis and Catalysis, 355(14-15), 2757–2763.
Cai, R., Chen, C., Li, Y., Sun, K., Zhou, F., Chen, K., & Jia, H. (2017). Improved soluble bacterial expression and properties of the recombinant flavonoid glucosyltransferase UGT73G1 from Allium cepa. Journal of Biotechnology, 255, 9–15.
He, X. Z., Li, W. S., & Blount, J. W. (2008). Regioselective synthesis of plant (iso) flavone glycosides in Escherichia coli. Applied Microbiology and Biotechnology, 80(2), 253–260.
Kim, J. H., Shin, K. H., & Ko, J. H. (2006). Glucosylation of flavonols by Escherichia coli expressing glucosyltransferase from rice (Oryza sativa). Journal of Bioscience and Bioengineering, 102(2), 135–137.
Sauerzapfe, B., Engels, L., & Elling, L. (2008). Broadening the biocatalytic properties of recombinant sucrose synthase 1 from potato (Solanum tuberosum L.) by expression in Escherichia coli and Saccharomyces cerevisiae. Enzyme Microb Tech, 43(3), 289–296.
Chen, L., Sun, P., Zhou, F., Li, Y., Chen, K., Jia, H., Yan, M., Gong, D., & Ouyang, P. (2018). Synthesis of rebaudioside D, using glycosyltransferase UGTSL2 and in situ UDP-glucose regeneration. Food Chemistry, 259, 286–291.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Biochemistry, 31(3), 426–428.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(s1–2), 248–254.
Ni, J., Gao, Y., Tao, F., Liu, H., & Xu, P. (2018). Temperature-directed biocatalysis for the sustainable production of aromatic aldehydes or alcohols. Angew Chem Int Edit, 57(5), 1214–1217.
Bedford, C. T., Hickman, A. D., & Logan, C. J. (2003). Structure-activity studies of glucose transfer: determination of the spontaneous rates of hydrolysis of uridine 5′-diphospho-α-D-glucose (UDPG) and uridine 5′-diphospho-α-D-glucuronic acid (UDPGA). Bioorgan Med Chem, 11(10), 2339–2345.
Wang, Y., Chen, L., Li, Y., Li, Y., Yan, M., Chen, K., Hao, N., & Xu, L. (2016). Efficient enzymatic production of rebaudioside A from stevioside. Bioscience Biotechnology and Biochemistry, 80(1), 67–73.
Weiz, G., Braun, L., Lopez, R., DeMaría, P. D., & Breccia, J. D. (2016). Enzymatic deglycosylation of flavonoids in deep eutectic solvents-aqueous mixtures: paving the way for sustainable flavonoid chemistry. J Mol Catal B-Enzym, 130, 70–73.
Mazzaferro, L. S., Piñuel, L., Erra-Balsells, R., Giudicessi, S. L., & Breccia, J. D. (2012). Transglycosylation specificity of Acremonium sp. α-rhamnosyl-β-glucosidase and its application to the synthesis of the new fluorogenic substrate 4-methylumbelliferyl-rutinoside. Carbohydrate Research, 347(1), 69–75.
Lim, E. K., Ashford, D. A., Hou, B., Jackson, R. G., & Bowles, D. J. (2004). Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotechnology and Bioengineering, 87(5), 623–631.
Lombard, K. A., Geoffriau, E., & Peffley, E. (2002). Flavonoid quantification in onion by spectrophotometric and high performance liquid chromatography analysis. HortScience, 37(4), 682–668.
Weignerova, L., Marhol, P., Gerstorferova, D., & Kren, V. (2012). Preparatory production of quercetin-3-beta-D-glucopyranoside using alkali-tolerant thermostable alpha-L-rhamnosidase from Aspergillus terreus. Bioresource Technology, 115, 222–227.
Zhang, R., Zhang, B. L., Xie, T., Li, G. C., Tuo, Y., & Xiang, Y. T. (2015). Biotransformation of rutin to isoquercitrin using recombinant rhamnosidase from Bifidobacterium breve. Biotechnology Letters, 37(6), 1257–1264.
Funding
This work was financially funded by the NSFC (21878155), PAPD, Qing Lan Project of Jiangsu Universities, Six Talent Peaks Project in Jiangsu Province, and Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture.
Author information
Authors and Affiliations
Contributions
RC, PS, and YL conceived and designed the study. RC, PS, and LC performed the experiments and analyzed the data. PS and RC wrote the paper. YL, HJ, KC, and MY reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ping Sun and Ruxin Cai are contributed to the work equally and should be regarded as co-first authors.
Electronic Supplementary Material
ESM 1
(DOC 228 kb)
Rights and permissions
About this article
Cite this article
Sun, P., Cai, R., Chen, L. et al. Natural Product Glycosylation: Biocatalytic Synthesis of Quercetin-3,4′-O-diglucoside. Appl Biochem Biotechnol 190, 464–474 (2020). https://doi.org/10.1007/s12010-019-03103-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03103-0