Abstract
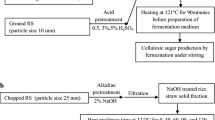
As one of the most abundant renewable resources, rice straw is an attractive lignocellulosic material for animal feeding or for the production of biochemical. An appropriate pre-treatment technique is essential for converting rice straw to rich fodder or biofuel. Based on previous work, Coprinopsis cinerea can grow on rice straw medium and therefore it is useful for the treatment of rice straw. However, little is known regarding its degradation systems and nutrition values. In this study, we firstly found that C. cinerea could grow rapidly on rice straw without any additives by the production of a series of enzymes (laccase, cellulase, and xylanase) and that the microstructure and contents of rice straw changed significantly after being treated by C. cinerea. We propose that a possible underlying mechanism exists in the degradation. Moreover, C. cinerea has a high nutrition value (23.5% crude protein and 22.2% total amino acids). Hence, fermented rice straw with mycelium could be a good animal feedstuff resource instead of expensive forage. The direct usage of C. cinerea treatment is expected to be a practical, cost-effective, and environmental-friendly approach for enhancing the nutritive value and digestibility of rice straw.



Similar content being viewed by others
References
Binod, P., Sindhu, R., Singhania, R. R., Vikram, S., Devi, L., Nagalakshmi, S., Kurien, N., Sukumaran, R. K., & Pandey, A. (2010). Bioethanol production from rice straw: an overview. Bioresource Technology, 101, 4767–4774.
Mussatto, S. I., & Roberto, I. C. (2004). Optimal experimental condition for hemicellulosic hydrolyzate treatment with activated charcoal for xylitol production. Biotechnology Progress, 20, 134–139.
Akinfemi, A., & Ogunwole, O. (2012). Chemical composition and in vitro digestibility of rice straw treated wih Pleurotus ostreatus, Pleurotus pulmonarius and Pleurotus tuber-regium. Slovak Journal of Animal Science, 45, 14–20.
Lim, J. S., Manan, Z. A., Alwi, S. R. W., & Hashim, H. (2012). A review on utilisation of biomass from rice industry as a source of renewable energy. Renewable and Sustainable Energy Reviews, 16, 3084–3094.
Yoswathana, N., Phuriphipat, P., Treyawutthiwat, P., & Eshtiaghi, M. N. (2010). Bioethanol production from rice straw. Energy Research Journal, 1, 26.
Alemahdi, N., Man, H. C., Abd Rahman, N., Nasirian, N., & Yang, Y. (2015). Enhanced mesophilic bio-hydrogen production of raw rice straw and activated sewage sludge by co-digestion. International Journal of Hydrogen Energy, 40, 16033–16044.
Kim, J. H., Block, D. E., Shoemaker, S. P., & Mills, D. A. (2010). Conversion of rice straw to bio-based chemicals: an integrated process using Lactobacillus brevis. Applied Microbiology and Biotechnology, 86, 1375–1385.
Van Soest, P. J. (2006). Rice straw, the role of silica and treatments to improve quality. Animal Feed Science and Technology, 130, 137–171.
Malik, K., Tokkas, J., & Kumari, R. C. A. A. N. (2015). Pretreated rice straw as an improved fodder for ruminants—an overview. Journal of Applied and Natural Science., 7, 514–520.
Ghasemi, E., Khorvash, M., Ghorbani, G. R., Emami, M. R., & Karimi, K. (2013). Dry chemical processing and ensiling of rice straw to improve its quality for use as ruminant feed. Tropical Animal Health and Production, 45, 1215–1221.
van Kuijk, S. J. A., Sonnenberg, A. S. M., Baars, J. J. P., Hendriks, W. H., & Cone, J. W. (2015). Fungal treated lignocellulosic biomass as ruminant feed ingredient: a review. Biotechnology Advances, 33, 191–202.
Kues, U. (2000). Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiology and Molecular Biology Reviews, 64, 316.
Zhou, Y., Zhang, W., Liu, Z., Wang, J., & Yuan, S. (2015). Purification, characterization and synergism in autolysis of a group of 1,3-beta-glucan hydrolases from the pilei of Coprinopsis cinerea fruiting bodies. Microbiology, 161, 1978–1989.
Moore, D., & Pukkila, P. J. (1985). Coprinus cinereus—an ideal organism for studies of genetics and developmental biology. Journal of Biological Education, 19, 31–40.
Mcdermid, K. P., Forsberg, C. W., & Mackenzie, C. R. (1990). Purification and properties of an acetylxylan esterase from Fibrobacter succinogenes S85. Applied and Environmental Microbiology, 56, 3805–3810.
Songulashvili, G., Elisashvili, V., Wasser, S. P., Nevo, E., & Hadar, Y. (2007). Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme and Microbial Technology, 41, 57–61.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Wood, T. M., & Bhat, K. M. (1988). Methods for measuring cellulase activities. Methods in Enzymology, 160, 87–112.
Zhang, W., Kang, L., Yang, M., Zhou, Y., Wang, J., Liu, Z., & Yuan, S. (2016). Purification, characterization and function analysis of an extracellular beta-glucosidase from elongating stipe cell walls in Coprinopsis cinerea. FEMS Microbiology Letters, 363, fnw120.
Tabka, M. G., Herpoel-Gimbert, I., Monod, F., Asther, M., & Sigoillot, J. C. (2006). Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzyme and Microbial Technology, 39, 897–902.
Vansoest, P. J., Robertson, J. B., & Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science, 74, 3583–3597.
AOAC. (1990). Official methods of analysis. Washington DC: Association of Official Analytical Chemists.
Nageh, A., El-masry, T., Elsayad, M., Shimaa, M. E., Sally, E., & Karima, I. (2015). Effects of rice straw burning products on guinea pig lungs. African Journal of Pharmacy and Pharmacology, 9, 645–661.
Zhang, W. M., Wu, X. X., Zhou, Y. J., Liu, Z. H., Zhang, W., Niu, X., Zhao, Y., Pei, S. Y., Zhao, Y., & Yuan, S. (2014). Characterization of stipe elongation of the mushroom Coprinopsis cinerea. Microbiology, 160, 1893–1902.
Liu, Z. H., Niu, X., Wang, J., Zhang, W. M., Yang, M. M., Liu, C. C., Xiong, Y. J., Zhao, Y., Pei, S. Y., Qin, Q., Zhang, Y., Yu, Y., & Yuan, S. (2015). Comparative study of nonautolytic mutant and wild-type strains of Coprinopsis cinerea supports an important role of glucanases in fruiting body autolysis. Journal of Agricultural and Food Chemistry, 63, 9609–9614.
Jalc, D., Nerud, F., & Siroka, P. (1998). The effectiveness of biological treatment of wheat straw by white-rot fungi. Folia Microbiologica, 43, 687–689.
Reddy, G. V., Babu, P. R., Komaraih, P., Roy, K. R. R. M., & Kothari, I. L. (2003). Utilization of banana waste for the production of lignolytic and cellulolytic enzymes by solid substrate fermentation using two Pleurotus species (P. ostreatus and P. sajorcaju). Process Biochemistry, 38, 1457–1462.
Rai, S. N., Walli, T. K., & Gupta, B. N. (1989). The chemical-composition and nutritive-value of rice straw after treatment with urea or Coprinus fimetarius in a solid-state fermentation system. Animal Feed Science and Technology, 26, 81–92.
Rahman, M. M., Lourenco, M., Hassim, H. A., Baars, J. J. P., Sonnenberg, A. S. M., Cone, J. W., De Boever, J., & Fievez, V. (2011). Improving ruminal degradability of oil palm fronds using white rot fungi. Animal Feed Science and Technology, 169, 157–166.
Okano, K., Kitagawa, M., Sasaki, Y., & Watanabe, T. (2005). Conversion of Japanese red cedar (Cryptomeria japonica) into a feed for ruminants by white-rot basidiomycetes. Animal Feed Science and Technology, 120, 235–243.
Asiegbu, F. O., Paterson, A., & Smith, J. E. (1996). The effects of co-fungal cultures and supplementation with carbohydrate adjuncts on lignin biodegradation and substrate digestibility. World Journal of Microbiology and Biotechnology, 12, 273–279.
Mukherjee, R., & Nandi, B. (2004). Improvement of in vitro digestibility through biological treatment of water hyacinth biomass by two Pleurotus species. International Biodeterioration & Biodegradation, 53, 7–12.
Bak, J. S., Ko, J. K., Choi, I. G., Park, Y. C., Seo, J. H., & Kim, K. H. (2009). Fungal pretreatment of lignocellulose by Phanerochaete chrysosporium to produce ethanol from rice straw. Biotechnology and Bioengineering, 104, 471–482.
Stajich, J. E., Wilke, S. K., Ahren, D., Au, C. H., Birren, B. W., Borodovsky, M., Burns, C., Canback, B., Casselton, L. A., Cheng, C. K., Deng, J. X., Dietrich, F. S., Fargo, D. C., Farman, M. L., Gathman, A. C., Goldberg, J., Guigo, R., Hoegger, P. J., Hooker, J. B., Huggins, A., James, T. Y., Kamada, T., Kilaru, S., Kodira, C., Kues, U., Kupfert, D., Kwan, H. S., Lomsadze, A., Li, W. X., Lilly, W. W., Ma, L. J., Mackey, A. J., Manning, G., Martin, F., Muraguchi, H., Natvig, D. O., Palmerini, H., Ramesh, M. A., Rehmeyer, C. J., Roe, B. A., Shenoy, N., Stanke, M., Ter-Hovhannisyan, V., Tunlid, A., Velagapudi, R., Vision, T. J., Zeng, Q. D., Zolan, M. E., & Pukkila, P. J. (2010). Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus). Proceedings of the National Academy of Sciences of the United States of America, 107, 11889–11894.
Wood, D. A. (1980). Inactivation of extracellular laccase during fruiting of Agaricus bisporus. Journal of General Microbiology, 117, 339–345.
Ross, I. K. (1982). The role of laccase in carpophore initiation in Coprinus congregatus. Journal of General Microbiology, 128, 2763–2770.
Dinis, M. J., Bezerra, R. M. F., Nunes, F., Dias, A. A., Guedes, C. V., Ferreira, L. M. M., Cone, J. W., Marques, G. S. M., Barros, A. R. N., & Rodrigues, M. A. M. (2009). Modification of wheat straw lignin by solid state fermentation with white-rot fungi. Bioresource Technology, 100, 4829–4835.
Iiyama, K., Lam, T. B. T., & Stone, B. A. (1994). Covalent cross-links in the cell-wall. Plant Physiology, 104, 315–320.
Nuchdang, S., Vatanyoopaisarn, S., & Phalakornkule, C. (2015). Effectiveness of fungal treatment by Coprinopsis cinerea and Polyporus tricholoma on degradation and methane yields of lignocellulosic grass. International Biodeterioration & Biodegradation, 104, 38–45.
Hatakka, A., & Hammel, K. E. (2011). Fungal biodegradation of lignocelluloses. In industrial applications (pp. 319–340). Berlin: Springer.
Chen, Y., Sharma-Shivappa, R. R., Keshwani, D., & Chen, C. (2007). Potential of agricultural residues and hay for bioethanol production. Applied Biochemistry and Biotechnology, 142, 276–290.
Reid, T., Munyanyi, M., & Mduluza, T. (2016). Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambiana. Food Science & Nutrition, 5, 538–544.
Cheung, P. C. (2008). Nutritional value and health benefits of mushrooms. In Mushrooms as Functional Foods (pp. 73–75). USA: John Wiley & Sons, Inc.
Liu, Y. T., Sun, J., Luo, Z. Y., Rao, S. Q., Su, Y. J., Xu, R. R., & Yang, Y. J. (2012). Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food and Chemical Toxicology, 50, 1238–1244.
Yin, J., & Zhou, L. (2008). Analysis of nutritional components of 4 kinds of wild edible fungi in Yunnan. Food Research and Development, 29, 133–136.
Vadiveloo, J. (2003). The effect of agronomic improvement and urea treatment on the nutritional value of Malaysian rice straw varieties. Animal Feed Science and Technology, 108, 133–146.
Selim, A. S. M., Pan, J., Takano, T., Suzuki, T., Koike, S., Kobayashi, Y., & Tanaka, K. (2004). Effect of ammonia treatment on physical strength of rice straw, distribution of straw particles and particle-associated bacteria in sheep rumen. Animal Feed Science and Technology, 115, 117–128.
Sarnklong, C., Cone, J. W., Pellikaan, W., & Hendriks, W. H. (2010). Utilization of rice straw and different treatments to improve its feed value for ruminants: a review. Asian Australasian Journal of Animal Sciences, 23, 680–692.
Devendra, C. (1997). Crop residues for feeding animals in Asia: Technology development and adoption in crop/livestock systems. In Crop residues in sustainable mixed crop/livestock farming systems (pp. 241–268). Wallingford, UK: CAB International.
Acknowledgements
The authors are thankful to Prof. Sheng Yuan, College of Life Science, Nanjing Normal University, China, for the gift of mushroom species. This work was supported by the “973” Program of China (Grant No. 2013CB733902), the National Natural Science Foundation of China (Grant No. 21306087), the Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20123221110008), the Program for New Century Excellent Talents in University, a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Program for Changjiang Scholars and Innovative Research Team in University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, W., Wu, S., Cai, L. et al. Improved Treatment and Utilization of Rice Straw by Coprinopsis cinerea . Appl Biochem Biotechnol 184, 616–629 (2018). https://doi.org/10.1007/s12010-017-2579-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2579-0