Abstract
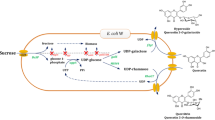
Escherichia coli strains expressing the O-glucosyltransferases UGT73B3 or UGT84B1 were compared for the production of glucosides from quercetin supplied into a defined medium. The formation of quercetin-3-glucoside (Q3G) by UGT73B3 showed a maximum at 33 °C, while the formation of quercetin-7-glucoside by UGT84B1 increased with increasing temperature to 37 °C. The highest concentrations of Q3G were attained by strains having a deletion in the pgi gene-coding phosphoglucose isomerase, which effectively blocked the entry of glucose-6P into the Embden–Meyerhof–Parnas pathway. Formation of Q3G was improved in 1-L controlled bioreactors compared to shake flask cultures, a result attributed to the greater oxygen transfer rate in bioreactors. Under batch conditions with 30 g/L glucose as the sole carbon source, E. coli MEC367 (MG1655 pgi) expressing UGT73B3 generated 3.9 g/L Q3G in 56 h.






Similar content being viewed by others
References
Hertog, M. G. L., Hollman, P. C. H., & van de Putte, B. (1993). Content of potentially anticarcinogenic flavonoids in tea infusions, wines and fruit juices. Journal of Agricultural and Food Chemistry, 41, 1242–1246.
Picinelli, A., Sua, B., & Mangas, J. J. (1997). Analysis of polyphenols in apple products. Zeitschrift für Lebensmittel-Untersuchung und Forschung A, 204, 48–51.
Price, K. R., & Rhodes, M. J. C. (1997). Analysis of the major flavonol glycosides present in four varieties of onion (Allium cepa) and changes in composition resulting from autolysis. Journal of the Science of Food and Agriculture, 74, 331–339.
Neveu, V., Perez-Jiménez, J., Vos, F., Crespy, V., du Chaffaut, L., Mennen, L., Knox, C., Eisner, R., Cruz, J., Wishart, D., and Scalbert, A. (2010). Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database.
Choi, E. J., Bae, S. M., & Ahn, W. S. (2008). Antiproliferative effects of quercetin through cell cycle arrest and apoptosis in human breast cancer MDA-MB-453 cells. Archives of Pharmacal Research, 31, 1281–1285.
Luo, H., Jiang, B. H., King, S. M., & Chen, Y. C. (2008). Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutrition and Cancer, 60, 800–809.
Jeong, J. H., An, J. Y., Kwon, Y. T., Rhee, J. G., & Lee, Y. J. (2009). Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. Journal of Cellular Biochemistry, 106, 73–82.
Robak, J., & Gryglewski, R. J. (1988). Flavonoids are scavengers of superoxide anions. Biochemical Pharmacology, 37, 837–841.
Inal, M. E., & Kahraman, A. (2000). The protective effect of flavonol quercetin against ultraviolet an induced oxidative stress in rats. Toxicology, 154, 21–29.
Duarte, J., Pérez-Palencia, R., Vargas, F., Ocete, M. A., Pérez-Vizcaíno, F., Zarzuelo, A., & Tamargo, J. (2001). Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. British Journal of Pharmacology, 133, 117–124.
Sánchez, M., Galisteo, M., Vera, R., Villar, I. C., Zarzuelo, A., Tamargo, J., Pérez-Vizcaíno, F., & Duarte, J. (2006). Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. Journal of Hypertension, 24, 75–84.
Yamamoto, Y., & Oue, E. (2006). Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Bioscience, Biotechnology and Biochemistry, 70, 933–939.
Gugler, R., Leschik, M., & Dengler, H. J. (1975). Disposition of quercetin in man after single oral and intravenous doses. European Journal of Clinical Pharmacology, 9, 229–234.
Makino, T., Shimizu, R., Kanemaru, M., Suzuki, Y., Moriwaki, M., & Mizukami, H. (2009). Enzymatically modified isoquercitrin, α-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biological and Pharmaceutical Bulletin, 32, 2034–2040.
Hollman, P. C., de Vries, J., van Leeuwen, S. D., Mengelere, M. J., & Katan, M. B. (1995). Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. American Journal of Clinical Nutrition, 62, 1276–1282.
Hollman, P. C., van der Gaag, M., Mengelers, M. J., van Trijp, J. M., de Vries, J. H., & Katan, M. B. (1996). Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radical Biology and Medicine, 21, 703–707.
Hollman, P. C., van Trijp, J. M., Buysman, M. N., van der Gaag, M. S., Mengelers, M. J., de Vries, J. H., & Katan, M. B. (1997). Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Letters, 418, 152–156.
Gee, J. M., DuPont, M. S., Day, A. J., Plumb, G. W., Willliamson, G., & Johnson, I. T. (2000). Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. Journal of Nutrition, 130, 2765–2771.
Crespy, V., Morand, C., Besson, C., Manach, C., Demigne, C., & Remesy, C. (2001). Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats. Journal of Nutrition, 131, 2109–2114.
Paulke, A., Eckert, G. P., Schubert-Zsilavecz, M., & Wurglics, M. (2012). Isoquercitrin provides better bioavailability than quercetin: comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Pharmazie, 67, 991–996.
Amado, N. G., Predes, D., Fonseca, B. F., Cerqueira, D. M., Reis, A. H., Dudenhoeffer, A. C., Borges, H. L., Mendes, F. A., & Abreu, J. G. (2014). Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. Journal of Biological Chemistry, 289, 35456–35467.
Day, A. J., Gee, J. M., DuPont, M. S., Johnson, I. T., & Williamson, G. (2003). Absorption of quercetin-3-glucoside and quercetin-4′-glycoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochemical Pharmacology, 65, 1199–1206.
Cermak, R., Landgraf, S., & Wolffram, S. (2004). Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. British Journal of Nutrition, 91, 849–855.
Song, J. H., Park, K. S., Kwon, D. H., & Choi, H. J. (2013). Anti-human rhinovirus 2 activity and mode of action of quercetin-7-glucoside from Lagerstroemia speciosa. Journal of Medicinal Food, 16, 274–279.
Lu, Z., Wang, J., Lin, S., & Zhan, Y. (2013). Degradation of rutin into isoquercitrin by Bacillus litoralis strain C44. IOSR Journal of Engineering, 2, 1154–1161.
De Bruyn, F., Maertens, J., Beauprez, J., Soetaert, W., & De Me, M. (2015). Biotechnological advances in UDP-sugar based glycosylation of small molecules. Biotechnology Advances, 33, 288–302.
He, X. Z., Li, W. S., Blount, J. W., & Dixon, R. A. (2008). Regioselective synthesis of plant (iso)flavone glycosides in Escherichia coli. Applied Microbiology and Biotechnology, 80, 253–260.
Kim, J. H., Shin, K. H., Ko, J. H., & Ahn, J. H. (2006). Glucosylation of flavonols by Escherichia coli expressing glucosyltransferase from rice (Oryza sativa). Journal of Bioscience and Bioengineering, 102, 135–137.
Lim, E. K., Ashford, D. A., Hou, B., Jackson, R. G., & Bowles, D. J. (2004). Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotechnology and Bioengineering, 87, 623–631.
Mao, Z., Shin, H. D., & Chen, R. R. (2006). Engineering the E. coli UDP-glucose synthesis pathway for oligosaccharide synthesis. Biotechnology Progress, 22, 369–374.
Lee, A. T., & Cerami, A. (1987). Elevated glucose 6-phosphate levels are associated with plasmid mutations in vivo. Proceedings of the National Academy of Sciences USA, 84, 8311–8314.
Morita, T., El-Kazzaz, W., Tanaka, Y., Inada, T., & Aiba, H. (2003). Accumulation of glucose 6-phosphate or fructose 6-phosphate is responsible for destabilization of glucose transporter mRNA in Escherichia coli. Journal of Biological Chemistry, 278, 15608–15614.
Yan, Y., Zhen, L., & Koffas, M. A. G. (2008). High-yield anthocyanin bioxynthesis in engineered Escherichia coli. Biotechnology and Bioengineering, 100, 126–140.
Lim, C. G., Wong, L., Bhan, N., Xu, P., Venkiteswaran, S., & Koffas, M. A. G. (2015). Development of a recombinant Escherichia coli strain for overproduction of the plant pigment anthocyanin. Applied and Environmental Microbiology, 81, 6276–6284.
Baba, T., Ara, T., Hasegawa, M., Takai, Y., Okumura, Y., Baba, M., Datsenko, K. A., Tomita, M., Wanner, B. L., & Mori, H. (2006). Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Molecular Systems Biology, 2, 1–11.
Datsenko, K. A., & Wanner, B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences USA, 97, 6640–6645.
Eiteman, M. A., & Chastain, M. J. (1997). Optimization of the ion-exchange analysis of organic acids from fermentation. Analytica et Chemica Acta, 338, 69–75.
St. John, A. C., Conklin, K., Rosenthal, E., & Goldberg, A. L. (1978). Further evidence for the involvement of charged tRNA and guanosine tetraphosphate in the control of protein degradation in Escherichia coli. Journal of Biological Chemistry, 253, 3945–3951.
Herendeen, S. L., van Bogelen, R. A., & Neidhardt, F. C. (1979). Levels of major proteins of Escherichia coli during growth at different temperatures. Journal of Bacteriology, 139, 185–194.
Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Biochemistry (5th ed.). New York: W H Freeman.
Li, Z., Nimtz, M., & Rinas, U. (2014). The metabolic potential of Escherichia coli BL21 in defined and rich medium. Microbial Cell Factories, 13, 1.
Chen, X., Alonso, A. P., Allen, D. K., Reed, J. L., & Shachar-Hill, Y. (2011). Synergy between 13C-metabolic flux analysis and flux balance analysis for understanding metabolic adaption to anaerobiosis in E. coli. Metabolic Engineering, 13, 38–48.
Canonaco, F., Hess, T. A., Heri, S., Wang, T., Szyperski, T., & Sauer, U. (2001). Metabolic flux response to phosphoglucose isomerase knockout in Escherichia coli and impact of overexpression of the soluble transhydrogenase UdhA. FEMS Microbiology Letters, 204, 247–252.
Fischer, E., & Sauer, U. (2003). Metabolic flux profiling of E. coli mutants in central carbon metabolism using GC-MS. European Journal of Biochemistry, 270, 880–891.
Zhao, J., Baba, T., Mori, H., & Shimizu, K. (2004). Effect of zwf gene knockout on the metabolism of Escherichia coli grown on glucose or acetate. Metabolic Engineering, 6, 164–174.
Olavarría, K., Valdés, D., & Cabrera, R. (2012). The cofactor preference of glucose-6-phosphate dehydrogenase from Escherichia coli—modeling the physiological production of reduced cofactors. FEBS Journal, 279, 2296–2309.
Yao, R., Hirose, Y., Sarkar, D., Nakahigashi, K., Ye, Q., & Shimizu, K. (2011). Catabolic regulation analysis of Escherichia coli and its crp, mlc, mgsA, pgi and ptsG mutants. Microbial Cell Factories, 10, 67.
Ramseier, T. M., Nègre, D., Cortay, J. C., Scarabel, M., Cozzone, A. J., & Saier, M. H. (1993). In vitro binding of the pleiotropic transcriptional regulatory protein, FruR, to the fru, pps, ace, pts and icd operons of Escherichia coli and Salmonella typhimurium. Journal of Molecular Biology, 234, 28–44.
Saier Jr., M. H., & Ramseier, T. M. (1996). The catabolite repressor/activator (Cra) protein of enteric bacteria. Journal of Bacteriology, 178, 3411–3417.
Shimada, T., Yamamoto, K., & Ishihama, A. (2011). Novel members of the Cra regulon involved in carbon metabolism in Escherichia coli. Journal of Bacteriology, 193, 649–659.
Kabir, M. M., & Shimizu, K. (2003). Gene expression patterns for metabolic pathway in pgi knockout Escherichia coli with and without phb genes based on RT-PCR. Journal of Biotechnology, 105, 11–31.
Hua, Q., Yang, C., Baba, T., Mori, H., & Shimizu, K. (2003). Responses of the central metabolism in Escherichia coli to phosphoglucose isomerase and glucose-6-phosphate dehydrogenase knockouts. Journal of Bacteriology, 185, 7053–7067.
Toya, Y., Ishii, N., Nakahigashi, K., Hirasawa, T., Soga, T., Tomita, M., & Shimizu, K. (2010). 13C-metabolic flux analysis for batch culture of Escherichia coli and its pyk and pgi gene knockout mutants based on mass isotopomer distribution of intracellular metabolites. Biotechnology Progress, 26, 975–992.
Usui, Y., Hirasawa, T., Furusawa, C., Shirai, T., Yamamoto, N., Mori, H., & Shimizu, H. (2012). Investigating the effects of perturbations to pgi and eno gene expression on central carbon metabolism in Escherichia coli using 13C metabolic flux analysis. Microbial Cell Factories, 11, 87.
Eiteman, M. A., & Altman, E. (2006). Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends in Biotechnology, 24, 530–536.
Brautaset, T., Petersen, S. B., & Valla, S. (1998). An experimental study on carbon flow in Escherichia coli as a function of kinetic properties and expression levels of the enzyme phosphoglucomutase. Biotechnology and Bioengineering, 5, 299–302.
Brautaset, T., Petersen, S. B., & Valla, S. (2000). In vitro determined kinetic properties of mutant phosphoglucomutases and their effects on sugar catabolism in Escherichia coli. Metabolic Engineering, 2, 104–114.
Lu, Q., Zhang, X., Almaula, N., Mathews, C. K., & Inouye, M. (1995). The gene for nucleoside diphosphate kinase functions as a mutator gene in Escherichia coli. Journal of Molecular Biology, 254, 337–341.
Leonard, E., Yan, Y., Fowler, Z. L., Li, Z., Lim, C. G., Lim, K. H., & Koffas, M. A. (2008). Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Molecular Pharmaceutics, 5, 257–265.
Acknowledgements
The authors thank Sarah Lee, Li Wang, and Don Armento for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, T., Eiteman, M.A. Quercetin Glucoside Production by Engineered Escherichia coli . Appl Biochem Biotechnol 182, 1358–1370 (2017). https://doi.org/10.1007/s12010-017-2403-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2403-x