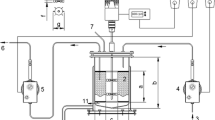
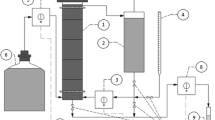
Abstract
An anaerobic sequencing batch reactor with immobilized biomass (AnSBBR) was applied to the production of biohydrogen treating a glucose-based wastewater. The influence of the applied volumetric organic load was studied by varying the concentration of influent at 3600 and 5250 mg chemical oxygen demand (COD) L−1 and cycle lengths of 4, 3, and 2 h resulting in volumetric organic loads of 10.5 to 31.1 g COD L−1. The results revealed system stability in the production of biohydrogen and substrate consumption. The best performance was an organic removal (COD) of 24 % and carbohydrate removal (glucose) of 99 %. Volumetric and specific molar productivity were 60.9 mol H2 m−3 day−1 and 5.8 mol H2 kg SVT−1 day−1 (biogas containing 40 % H2 and no CH4) at 20.0 g COD L−1 day−1 (5250 mg COD L−1 and 3 h). The yield between produced hydrogen and removed organic matter in terms of carbohydrates was 0.94 mol H2 Mol GLU−1 (biogas containing 52 % H2 and no CH4) at 10.5 g COD L−1 day−1 (3600 mg COD L−1 and 4 h), corresponding to 23 and 47 % of the theoretical values of the acetic and butyric acid metabolic routes, respectively. Metabolites present at significant amounts were ethanol, acetic acid, and butyric acid. The conditions with higher influent concentration and intermediate cycle length, and the condition with lower influent concentration and longer cycle showed the best results in terms of productivity and yield, respectively. This indicates that the best productivity tends to occur at higher organic loads, as this parameter involves the biogas production, and the best yield tends to occur at lower and/or intermediate organic loads, as this parameter also involves substrate consumption.






Similar content being viewed by others
Abbreviations
- ASOLCT :
-
Applied specific organic load based on organic matter—nonfiltered sample [kg COD m−3 day−1]
- ASOLST :
-
Applied specific organic load based on carbohydrate (glucose)—nonfiltered sample [kg GLUC m−3 day−1]
- AVOLCT :
-
Applied volumetric organic load based on organic matter—nonfiltered sample [kg COD m−3 day−1]
- AVOLST :
-
Applied volumetric organic load based on carbohydrate (glucose)—nonfiltered sample [kg GLU m−3 day−1]
- BA:
-
Bicarbonate alkalinity [mg CaCO3 L−1]
- CCF :
-
Concentration based on organic matter for filtered samples in the effluent [mg COD L−1]
- CCT :
-
Concentration based on organic matter for unfiltered samples in the effluent [mg COD L−1]
- CCT,I :
-
Concentration based on organic matter for unfiltered samples in the influent [mg COD L−1]
- CH2 :
-
Concentration of hydrogen [mmol L−1]
- CSF :
-
Concentration based on carbohydrates (glucose) for filtered samples in the effluent [mg GLU L−1 or mmol GLU L−1]
- CST :
-
Concentration based on carbohydrates (glucose) for unfiltered samples in the effluent [mg GLU L−1]
- CST,I :
-
Concentration based on carbohydrates (glucose) for unfiltered samples in the influent [mg GLU L−1]
- CCF :
-
Concentration based on organic matter for filtered samples [mg COD L−1]
- CX-TVS :
-
Concentration of biomass in the reactor in total volatile solids per volume of liquid [g TVS L−1]
- C′X-TVS :
-
Concentration of biomass in the reactor in total volatile solids per mass of support [g TVS gsupport −1]
- MTVS :
-
Total biomass in the reactor in total volatile solids [g TVS]
- MPr:
-
Daily molar productivity of hydrogen [mol H2 m−3 day−1]
- MYALC,m :
-
Molar yield per applied load based on organic matter expressed as kilograms [mol H2 kg COD−1]
- MYALC,n :
-
Molar yield per applied load based on organic matter expressed as moles [mol H2 mol COD−1]
- MYALS,m :
-
Molar yield per applied load based on carbohydrates (glucose) expressed as kilograms [mol H2 kg GLU−1]
- MYALS,n :
-
Molar yield per applied load based on carbohydrates (glucose) expressed as moles [mol H2 mol GLU−1]
- MYRLC,m :
-
Molar yield per removed load based on organic matter expressed as kilograms [mol H2 kg COD−1]
- MYRLC,n :
-
Molar yield per removed load based on organic matter expressed as moles [mol H2 mol COD−1]
- MYRLS,m :
-
Molar yield per removed load based on carbohydrates (glucose) expressed as kilograms [mol H2 kg GLU−1]
- MYRLS,n :
-
Molar yield per removed load based on carbohydrates (glucose) expressed as moles [mol H2 mol GLU−1]
- NG :
-
Molar quantity of biogas (H2, CO2, and CH4) produced along a cycle (mmol)
- nH2 :
-
Daily molar production of hydrogen [mol day−1]
- RSOLCF :
-
Removed volumetric specific load based on organic matter—filtered sample [kg COD g TVS−1 day−1]
- RSOLSF :
-
Removed volumetric specific load based on carbohydrate (glucose)—filtered sample [kg GLU g TVS−1 day−1]
- RVOLCF :
-
Removed specific organic load based on organic matter—filtered sample [kg COD m−3 day−1]
- RVOLSF :
-
Removed specific organic load based on carbohydrates (glucose)—filtered sample [kg GLU m−3 day−1]
- SMPr:
-
Daily specific molar productivity of hydrogen [mol H2 kg TVS−1 day−1]
- tC :
-
Cycle length [h cycle−1]
- TS:
-
Total solids concentration [mg L−1]
- TSS:
-
Total suspended solids concentration [mg L−1]
- TVA:
-
Total volatile acids [mg HAc L−1]
- TVS:
-
Total volatile solids concentration [mg L−1]
- VSS:
-
Volatile suspended solids concentration [mg L−1]
- VF :
-
Volume of wastewater fed during the cycle [L cycle−1]
- VG :
-
Normal volume of biogas (H2, CO2, and CH4) produced along a cycle (N mL)
- VR :
-
Volume of liquid medium in the reactor [L]
- εCF :
-
Removal efficiency based on organic matter for filtered samples [%]
- εCT :
-
Removal efficiency based on organic matter for unfiltered samples [%]
- εSF :
-
Removal efficiency based on carbohydrates (glucose) for filtered samples [%]
- εST :
-
Removal efficiency based on carbohydrates (glucose) for unfiltered samples [%]
- HAc:
-
Acetic acid
- HBut:
-
Butyric acid
- HPr:
-
Propionic acid
- EtOH:
-
Ethanol
- vSF :
-
Carbohydrate consumption rate for filtered samples [mmol L−1 h−1]
- vH2 :
-
Hydrogen formation rate [mmol L−1 h−1]
References
Kapdan, K. I., & Kargi, F. (2006). Bio-hydrogen production from waste materials. Enzyme and Microbial Technology, 38, 569–582.
Das, D., & Veziroglu, T. N. (2001). Hydrogen production by biological processes: a survey of literature. International Journal of Hydrogen Energy, 26, 13–28.
Lin, C., & Lay, C. (2004). Carbon/nitrogen ratio effect on fermentative hydrogen production by mixed microflora. International Journal of Hydrogen Energy, 29, 41–45.
Lin, C., & Lay, C. (2004). Effects of carbonate and phosphate concentrations on hydrogen production using anaerobic sewage sludge microflora. International Journal of Hydrogen Energy, 29, 275–281.
Lin, C., & Lay, C. (2005). A nutrient formulation for fermentative hydrogen production using anaerobic sewage sludge microflora. International Journal of Hydrogen Energy, 30, 285–292.
Kawagoshi, Y., Hino, N., & Fujimoto, A. (2005). Effect of inoculum conditioning on hydrogen fermentation and pH effect on bacterial community relevant to hydrogen production. Journal of Bioscience and Bioengineering, 100, 524–530.
Li, C., & Fang, H. (2007). Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Critical Reviews in Environmental Science and Technology, 37, 1–39.
Arooj, M., Han, S., Kim, S., Kim, D., & Shin, H. (2008). Effect of HRT on ASBR converting starch into biological hydrogen. International Journal of Hydrogen Energy, 33, 6509–6514.
Turcot, J., Bisaillon, A., & Hallenbeck, P. (2008). Hydrogen production by continuous cultures of Escherichia coli under different nutrient regimes. International Journal of Hydrogen Energy, 33, 1465–1470.
Davila-Vazquez, G., Arriaga, S., & Alatriste-Mondragón, F. (2007). Fermentative biohydrogen production: trends and perspectives. Reviews in Environmental Science and Bio/Technology, 7, 27–45.
Ren, N., Guo, W., & Wang, X. (2008). Effects of different pretreatment methods on fermentation types and dominant bacteria for hydrogen production. International Journal of Hydrogen Energy, 33, 4318–4324.
Wang, X., Monis, P. T., Saint, C. P., & Jin, B. (2009). Biochemical kinetics of fermentative hydrogen production by Clostridium butyricum W5. International Journal of Hydrogen Energy, 34, 791–798.
Wang, J., & Wan, W. (2009). Factors influencing fermentative hydrogen production: a review. International Journal of Hydrogen Energy, 34, 799–811.
Mu, Y., Zheng, X., Yu, H., & Zhu, R. (2006). Biological hydrogen production by anaerobic sludge at various temperatures. International Journal of Hydrogen Energy, 31, 780–785.
Bhaskar, Y. V., Mohan, S. V., & Sarma, P. N. (2008). Effect of substrate loading rate of chemical wastewater on fermentative biohydrogen production in biofilm configured sequencing batch reactor. Bioresource Technology, 99, 6941–6948.
Santos, D. A., Rodrigues, J. A. D., Ratusznei, S., & Zaiat, M. (2014). AnSBBR with circulation applied to biohydrogen production treating sucrose-based wastewater: effects of organic loading, influent concentration and cycle length. Brazilian Journal of Chemical Engineering, 31, 659–674.
Manssouri, M., Rodrigues, J. A. D., Ratusznei, S. M., & Zaiat, M. (2013). Effects of organic loading, influent concentration and feed time on biohydrogen production in a mechanically stirred AnSBBR treating sucrose-based wastewater. Applied Biochemistry and Biotechnology, 171, 1832–1854.
Dubois, S. M., Gilles, K. A., Hamilton, J. L., Rebers, P. A., & Smith, F. (1956). Colorimetric Methods for determination of sugar and related substance. Analytical Chemistry, 228, 13–21.
Standard Methods for the Examination of Water and Wastewater. (1995). APHA, AWWA, WPCF (19th ed.). Washington: American Public Health Association.
Acknowledgments
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (São Paulo, Brasil), process numbers 09/15.984-0, 12.01.039-5 (L.P. Souza), and 12/01.048-4 (T.G. Lullio). The authors gratefully acknowledge Dr. Baltus C. Bonse for the revision of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souza, L.P., Lullio, T.G., Ratusznei, S.M. et al. Influence of Organic Load on Biohydrogen Production in an AnSBBR Treating Glucose-Based Wastewater. Appl Biochem Biotechnol 176, 796–816 (2015). https://doi.org/10.1007/s12010-015-1612-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1612-4