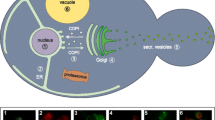
Abstract
Pichia pastoris has currently been developed as an effective host system for the expression of heterologous genes owing to its potential use for the production of soluble and high-yield proteins. However, the secretory production of the different heterologous proteins in P. pastoris varies widely. Some factors restrict the effective secretory production of heterologous proteins in P. pastoris, among which the folding and processing of proteins is a major one. Besides optimizing the fermentative process, current strategies focus on investigating protein folding process. Thus, this paper is the first time to review the improvement of the secretory production of the heterologous protein in P. pastoris by focusing on its folding process.


Similar content being viewed by others
References
Cereghino, G. P. L., Cereghino, J. L., Ilgen, C., & Cregg, J. M. (2002). Production of recombinant proteins in fermenter cultures of the yeast Pichia pastoris. Current Opinion in Biotechnology, 13, 329–332.
Daly, R., & Hearn, M. T. (2005). Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. Journal of Molecular Recognition, 18, 119–138.
Cregg, J., Cereghino, J., Shi, J., & Higgins, D. (2000). Recombinant protein expression in Pichia pastoris. Molecular Biotechnology, 16, 23–52.
Cereghino, J. L., & Cregg, J. M. (2000). Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiology Reviews, 24, 45–66.
Cereghino, G., Sunga, A., Cereghino, J., & Cregg, J. (2002). Expression of foreign genes in the yeast Pichia pastoris: principles and methods.
Yu, P. (2007). A new approach to the production of the recombinant SOD protein by methylotrophic Pichia pastoris. Applied Microbiology and Biotechnology, 74, 93–98.
De Schutter, K., Lin, Y. C., Tiels, P., Van Hecke, A., Glinka, S., Weber-Lehmann, J., Rouze, P., Van de Peer, Y., & Callewaert, N. (2009). Genome sequence of the recombinant protein production host Pichia pastoris. Nature Biotechnology, 27, 561–566.
Porro, D., Sauer, M., Branduardi, P., & Mattanovich, D. (2005). Recombinant protein production in yeasts. Molecular Biotechnology, 31, 245–259.
Schröder, M. (2008). Engineering eukaryotic protein factories. Biotechnology Letters, 30, 187–196.
Abad, S., Kitz, K., Hörmann, A., Schreiner, U., Hartner, F. S., & Glieder, A. (2010). Real-time PCR-based determination of gene copy numbers in Pichia pastoris. Biotechnology Journal, 5, 413–420.
Sørensen, H. P., & Mortensen, K. K. (2005). Advanced genetic strategies for recombinant protein expression in Escherichia coli. Journal of Biotechnology, 115, 113–128.
Zhang, W., Zhao, H. L., Xue, C., Xiong, X. H., Yao, X. Q., Li, X. Y., Chen, H. P., & Liu, Z. M. (2006). Enhanced secretion of heterologous proteins in Pichia pastoris following overexpression of Saccharomyces cerevisiae chaperone proteins. Biotechnology Progress, 22, 1090–1095.
Gasser, B., Sauer, M., Maurer, M., Stadlmayr, G., & Mattanovich, D. (2007). Transcriptomics-based identification of novel factors enhancing heterologous protein secretion in yeasts. Applied and Environmental Microbiology, 73, 6499–6507.
Xu, P., & Robinson, A. S. (2009). Decreased secretion and unfolded protein response up-regulation are correlated with intracellular retention for single-chain antibody variants produced in yeast. Biotechnology and Bioengineering, 104, 20–29.
Anelli, T., & Sitia, R. (2008). Protein quality control in the early secretory pathway. The EMBO Journal, 27, 315–327.
Buck, T. M., Wright, C. M., & Brodsky, J. L. (2007). The activities and function of molecular chaperones in the endoplasmic reticulum. Seminars in Cell and Developmental Biology, 18, 751–761.
Meunier, L., Usherwood, Y. K., Chung, K. T., & Hendershot, L. M. (2002). A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Molecular Biology of the Cell, 13, 4456–4469.
Sitia, R., & Braakman, I. (2003). Quality control in the endoplasmic reticulum protein factory. Nature, 426, 891–894.
Kleizen, B., & Braakman, I. (2004). Protein folding and quality control in the endoplasmic reticulum. Current Opinion in Cell Biology, 16, 343–349.
Nishikawa, S., Fewell, S. W., Kato, Y., Brodsky, J. L., & Endo, T. (2001). Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. Journal of Cell Biology, 153, 1061–1070.
Schröder, M., & Kaufman, R. J. (2005). ER stress and the unfolded protein response. Mutation Research-Fundmental and Molecular Mechanisms of Mutagensis, 569, 29–63.
Dorner, A. J., & Kaufman, R. J. (1990). In: V. G. David (ed.), Method enzymol, vol. Volume 185 (pp. 577–596) Academic Press.
Shen, Y., Chung, K. T., & Hendershot, L. M. (2008). In: Protein science encyclopedia, Wiley-VCH Verlag GmbH & Co. KGaA.
Harald, W., Sebastian, K. W., Andreas, B. S., Jochen, R., & Johannes, B. (2006). Substrate transfer from the chaperone Hsp70 to Hsp90. Journal of Molecular Biology, 356, 802–811.
Inan, M., Aryasomayajula, D., Sinha, J., & Meagher, M. M. (2006). Enhancement of protein secretion in Pichia pastoris by overexpression of protein disulfide isomerase. Biotechnology and Bioengineering, 93, 771–778.
Zhang, L., Chou, C. P., & Moo-Young, M. (2011). Disulfide bond formation and its impact on the biological activity and stability of recombinant therapeutic proteins produced by Escherichia coli expression system. Biotechnology Advances, 29, 923–929.
Tu, B. P., & Weissman, J. S. (2002). The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Molecular Cell, 10, 983–994.
Wang, W. Z., Jakob, R. W., & Colin, T. (2007). Erv2p: characterization of the redox behavior of a yeast sulfhydryl oxidase. Biochemistry, 46, 3246–3254.
Bretthauer, R. K., & Castellino, F. J. (1999). Glycosylation of Pichia pastoris-derived proteins. Biotechnology and Applied Biochemistry, 30, 193–200.
Arnold, J. N., Wormald, M. R., Sim, R. B., Rudd, P. M., & Dwek, R. A. (2007). The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual Reviews of Immunology, 25, 21–50.
Yoshida, H. (2007). ER stress and diseases. The FEBS Journal, 274, 630–658.
Damasceno, L. M., Anderson, K. A., Ritter, G., Cregg, J. M., Old, L. J., & Batt, C. A. (2007). Cooverexpression of chaperones for enhanced secretion of a single-chain antibody fragment in Pichia pastoris. Applied Microbiology and Biotechnology, 74, 381–389.
Gasser, B., Saloheimo, M., Rinas, U., Dragosits, M., Rodríguez-Carmona, E., Baumann, K., Giuliani, M., Parrilli, E., Branduardi, P., & Lang, C. (2008). Protein folding and conformational stress in microbial cells producing recombinant proteins: a host comparative overview. Microbial Cell Factories, 7, 11.
Cereghino, G. P. L., & Cregg, J. M. (1999). Applications of yeast in biotechnology: protein production and genetic analysis. Current Opinion in Biotechnology, 10, 422–427.
Butz, J. A., Niebauer, R. T., & Robinson, A. S. (2003). Co-expression of molecular chaperones does not improve the heterologous expression of mammalian G-protein coupled receptor expression in yeast. Biotechnology and Bioengineering, 84, 292–304.
Okuda-Shimizu, Y., & Hendershot, L. M. (2007). Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require herp. Molecular Cell, 28, 544–554.
Payne, T., Finnis, C., Evans, L. R., Mead, D. J., Avery, S. V., Archer, D. B., & Sleep, D. (2008). Modulation of chaperone gene expression in mutagenized Saccharomyces cerevisiae strains developed for recombinant human albumin production results in increased production of multiple heterologous proteins. Applied and Environmental Microbiology, 74, 7759–7766.
Xu, P., Raden, D., Doyle Iii, F. J., & Robinson, A. S. (2005). Analysis of unfolded protein response during single-chain antibody expression in Saccaromyces cerevisiae reveals different roles for BiP and PDI in folding. Metabolic Engineering, 7, 269–279.
Humphreys, D. P., Weir, N., Lawson, A., Mountain, A., & Lund, P. A. (1996). Co-expression of human protein disulphide isomerase (PDI) can increase the yield of an antibody Fab’ fragment expressed in Escherichia coli. FEBS Letters, 380, 194–197.
Mehmet, I., Sarah, A. F., Zhang, W. H. H. P. J., Zhan, B., & Michael, M. M. (2007). Pichia protocols. In J. M. Cregg (Ed.), Methods in molecular biology (389th ed., pp. 65–75). New York: Humana Press.
Li, Z., Moy, A., Gomez, S. R., Franz, A. H., Lin-Cereghino, J., & Lin-Cereghino, G. P. (2010). An improved method for enhanced production and biological activity of human secretory leukocyte protease inhibitor (SLPI) in Pichia pastoris. Biochemical and Biophysical Research Communications, 402, 519–524.
Vad, R., Nafstad, E., Dahl, L. A., & Gabrielsen, O. S. (2005). Engineering of a Pichia pastoris expression system for secretion of high amounts of intact human parathyroid hormone. Journal of Biotechnology, 116, 251–260.
Ron, D., & Walter, P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology, 8, 519–529.
Damasceno, L., Huang, C., Jr., & Batt, C. (2012). Protein secretion in Pichia pastoris and advances in protein production. Applied Microbiology and Biotechnology, 93, 31–39.
Guerfal, M., Ryckaert, S., Jacobs, P. P., Ameloot, P., Van Craenenbroeck, K., Derycke, R., & Callewaert, N. (2010). The HAC1 gene from Pichia pastoris: characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microbial Cell Factories, 49–61.
Ng, D. T. W., Spear, E. D., & Walter, P. (2000). The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control. Journal of Cell Biology, 150, 77–88.
Graf, A., Gasser, B., Dragosits, M., Sauer, M., Leparc, G., Tuchler, T., Kreil, D., & Mattanovich, D. (2008). Novel insights into the unfolded protein response using Pichia pastoris specific DNA microarrays. BMC Genomics, 9, 390.
Valkonen, M., Penttilä, M., & Saloheimo, M. (2003). Effects of inactivation and constitutive expression of the unfolded-protein response pathway on protein production in the yeast Saccharomyces cerevisiae. Applied and Environmental Microbiology, 69, 2065–2072.
Gasser, B., Maurer, M., Gach, J., Kunert, R., & Mattanovich, D. (2006). Engineering of Pichia pastoris for improved production of antibody fragments. Biotechnology and Bioengineering, 94, 353–361.
Goochee, C. F., Gramer, M. J., Andersen, D. C., Bahr, J. B., & Rasmussen, J. R. (1991). The oligosaccharides of glycoproteins: bioprocess factors affecting oligosaccharide structure and their effect on glycoprotein properties. Nature Biotechnology, 9, 1347–1355.
Valentinis, B., Bhala, A., DeAngelis, T., Baserga, R., & Cohen, P. (1995). The human insulin-like growth factor (IGF) binding protein-3 inhibits the growth of fibroblasts with a targeted disruption of the IGF-I receptor gene. Molecular Endocrinology, 9, 361–367.
Juge, N., Andersen, J. S., Tull, D., Roepstorff, P., & Svensson, B. (1996). Overexpression, purification, and characterization of recombinant barley α-amylases 1 and 2 secreted by the methylotrophic yeast Pichia pastoris. Protein Expression and Purification, 8, 204–214.
Heimo, H., Palmu, K., & Suominen, I. (1997). Expression in Pichia pastoris and purification of Aspergillus awamori glucoamylase catalytic domain. Protein Expression and Purification, 10, 70–79.
Tsujikawa, M., Okabayashi, K., Morita, M., & Tanabe, T. (1996). Secretion of a variant of human single-chain urokinase-type plasminogen activator without an N-glycosylation site in the methylotrophic yeast, Pichia pastoris and characterization of the secreted product. Yeast, 12, 541–553.
Duman, J. G., Miele, R. G., Liang, H., Grella, D. K., Sim, K. L., Castellino, F. J., & Bretthauer, R. K. (1998). O-Mannosylation of Pichia pastoris cellular and recombinant proteins. Biotechnology and Applied Biochemistry, 28, 39–45.
Gemmill, T. R., & Trimble, R. B. (1999). Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochimica et Biophysica Acta (BBA)-General Subjects, 1426, 227–237.
Oka, T., & Jigami, Y. (2006). Reconstruction of de novo pathway for synthesis of UDP-glucuronic acid and UDP-xylose from intrinsic UDP-glucose in Saccharomyces cerevisiae. FEBS Journal, 273, 2645–2657.
Chigira, Y., Oka, T., Okajima, T., & Jigami, Y. (2008). Engineering of a mammalian O-glycosylation pathway in the yeast Saccharomyces cerevisiae: production of O-fucosylated epidermal growth factor domains. Glycobiology, 18, 303–314.
Idiris, A., Tohda, H., Kumagai, H., & Takegawa, K. (2010). Engineering of protein secretion in yeast: strategies and impact on protein production. Applied Microbiology and Biotechnology, 86, 403–417.
Ikeda, Y., Ohashi, T., Tanaka, N., & Takegawa, K. (2009). Identification and characterization of a gene required for α-1, 2-mannose extension in the O-linked glycan synthesis pathway in Schizosaccharomyces pombe. FEMS Yeast Research, 9, 115–125.
Ohashi, T., & Takegawa, K. (2010). N-and O-linked oligosaccharides completely lack galactose residues in the gms1och1 mutant of Schizosaccharomyces pombe. Applied Microbiology and Biotechnology, 86, 263–272.
Montesino, R., Garcia, R., Quintero, O., & Cremata, J. A. (1998). Variation in N-Linked oligosaccharide structures on heterologous proteins secreted by the methylotrophic yeast Pichia pastoris. Protein Expression and Purification, 14, 197–207.
Trimble, R. B., Atkinson, P. H., Tschopp, J. F., Townsend, R. R., & Maley, F. (1991). Structure of oligosaccharides on Saccharomyces SUC2 invertase secreted by the methylotrophic yeast Pichia pastoris. Journal of Biological Chemistry, 266, 22807–22817.
Miele, R. G., Castellino, F. J., & Bretthauer, R. K. (1997). Characterization of the acidic oligosaccharides assembled on the Pichia pastoris-expressed recombinant kringle 2 domain of human tissue-type plasminogen activator. Biotechnology and Applied Biochemistry, 26, 79–83.
Montesino, R., Cremata, J., Rodriguez, M., Besada, V., Falcon, V., & La Fuente, J. (1996). Biochemical characterization of the recombinant Boophilus microplus Bm86 antigen expressed by transformed Pichia pastoris cells. Biotechnology and Applied Biochemistry, 23, 23–28.
Verostek, M. F., & Trimble, R. B. (1995). Mannosyltransferase activities in membranes from various yeast strains. Glycobiology, 5, 671–681.
Hamilton, S. R., & Gerngross, T. U. (2007). Glycosylation engineering in yeast: the advent of fully humanized yeast. Current Opinion in Biotechnology, 18, 387–392.
Chiba, Y., & Akeboshi, H. (2009). Glycan engineering and production of ‘humanized’ glycoprotein in yeast cells. Biological and Pharmaceutical Bulletin, 32, 786–795.
Chiba, Y., Suzuki, M., Yoshida, S., Yoshida, A., Ikenaga, H., Takeuchi, M., Jigami, Y., & Ichishima, E. (1998). Production of human compatible high mannose-type (Man5GlcNAc2) sugar chains in Saccharomyces cerevisiae. Journal of Biological Chemistry, 273, 26298–26304.
Nakanishi-Shindo, Y., Nakayama, K. I., Tanaka, A., Toda, Y., & Jigami, Y. (1993). Structure of the N-linked oligosaccharides that show the complete loss of alpha-1, 6-polymannose outer chain from och1, och1 mnn1, and och1 mnn1 alg3 mutants of Saccharomyces cerevisiae. Journal of Biological Chemistry, 268, 26338–26345.
Hamilton, S. R., Bobrowicz, P., Bobrowicz, B., Davidson, R. C., Li, H., Mitchell, T., Nett, J. H., Rausch, S., Stadheim, T. A., & Wischnewski, H. (2003). Production of complex human glycoproteins in yeast. Science, 301, 1244–1246.
Hamilton, S. R., Davidson, R. C., Sethuraman, N., Nett, J. H., Jiang, Y., Rios, S., Bobrowicz, P., Stadheim, T. A., Li, H., & Choi, B. K. (2006). Humanization of yeast to produce complex terminally sialylated glycoproteins. Science, 313, 1441–1443.
Wentz, A. E., & Shusta, E. V. (2007). A novel high-throughput screen reveals yeast genes that increase secretion of heterologous proteins. Applied and Environmental Microbiology, 73, 1189–1198.
Seung Bum, S., Alexandra, B. G., Tae Yong, K., Brigitte, G., Michael, M., Pau, F., Diethard, M., & Sang Yup, L. (2010). Genome-scale metabolic model of methylotrophic yeast Pichia pastoris and its use for in silico analysis of heterologous protein production. Biotechnology Journal, 5, 705–715.
Alexandra, G., Martin, D., Brigitte, G., & Diethard, M. (2009). Yeast systems biotechnology for the production of heterologous proteins. FEMS Yeast Research, 9, 335–348.
Dragosits, M., Stadlmann, J., Albiol, J., Baumann, K., Maurer, M., Gasser, B., Sauer, M., Altmann, F., Ferrer, P., & Mattanovich, D. (2009). The effect of temperature on the proteome of recombinant Pichia pastoris. Journal of Proteome Research, 8, 1380–1392.
Gasser, B., Maurer, M., Rautio, J., Sauer, M., Bhattacharyya, A., Saloheimo, M., Penttila, M., & Mattanovich, D. (2007). Monitoring of transcriptional regulation in Pichia pastoris under protein production conditions. BMC Genomics, 8, 179.
Stadlmayr, G., Benakovitsch, K., Gasser, B., Mattanovich, D., & Sauer, M. (2010). Genome-scale analysis of library sorting (GALibSo): isolation of secretion enhancing factors for recombinant protein production in Pichia pastoris. Biotechnology and Bioengineering, 105, 543–555.
Shusta, E. V., Raines, R. T., Plückthun, A., & Wittrup, K. D. (1998). Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nature Biotechnology, 16, 773–777.
Werten, M. W., & de Wolf, F. A. (2005). Reduced proteolysis of secreted gelatin and Yps1-mediated α-factor leader processing in a Pichia pastoris Kex2 disruptant. Applied and Environmental Microbiology, 71, 2310–2317.
Gross, E., Kastner, D. B., Kaiser, C. A., & Fass, D. (2004). Structure of Ero1p, source of disulfide bonds for oxidative protein folding in the cell. Cell, 117, 601–610.
Bonander, N., Darby, R. A., Grgic, L., Bora, N., Wen, J., Brogna, S., Poyner, D. R., O’Neill, M. A., & Bill, R. M. (2009). Altering the ribosomal subunit ratio in yeast maximizes recombinant protein yield. Microbial Cell Factories, 8, 10.
Rakestraw, J. A., Baskaran, A. R., & Wittrup, K. D. (2006). A flow cytometric assay for screening improved heterologous protein secretion in yeast. Biotechnology Progress, 22, 1200–1208.
Bonander, N., Hedfalk, K., Larsson, C., Mostad, P., Chang, C., Gustafsson, L., & Bill, R. M. (2005). Design of improved membrane protein production experiments: quantitation of the host response. Protein Science, 14, 1729–1740.
Sauer, M., Branduardi, P., Gasser, B., Valli, M., Maurer, M., Porro, D., & Mattanovich, D. (2004). Differential gene expression in recombinant Pichia pastoris analysed by heterologous DNA microarray hybridisation. Microbial Cell Factories, 3, 17.
Giga-Hama, Y., Tohda, H., Takegawa, K., & Kumagai, H. (2007). Schizosaccharomyces pombe minimum genome factory. Biotechnology and Applied Biochemistry, 46, 147–155.
Takegawa, K., Tokudomi, S., Bhuiyan, M. S. A., Tabuchi, M., Fujita, Y., Iwaki, T., Utsumi, S., & Tanaka, N. (2003). Heterologous expression and characterization of Schizosaccharomyces pombe vacuolar carboxypeptidase Y in Saccharomyces cerevisiae. Current Genetics, 42, 252–259.
Mukaiyama, H., Giga-Hama, Y., Tohda, H., & Takegawa, K. (2009). Dextran sodium sulfate enhances secretion of recombinant human transferrin in Schizosaccharomyces pombe. Applied Microbiology and Biotechnology, 85, 155–164.
Kobayashi, K., Kuwae, S., Ohya, T., Ohda, T., Ohyama, M., Ohi, H., Tomomitsu, K., & Ohmura, T. (2000). High-level expression of recombinant human serum albumin from the methylotrophic yeast Pichia pastoris with minimal protease production and activation. Journal of Bioscience and Bioengineering, 89, 55–61.
Yang, Y. F., Yuan, H. Y., Liu, N. S., Chen, X., Gao, B., Lu, H., & Li, Y. (2005). Construction, expression and characterization of human interferon α2b-(G4S) n-thymosin α1 fusion proteins in Pichia pastoris. World Journal of Gastroenterology, 11, 2597–2602.
Skoko, N., Argamante, B., Grujičić, N. K., Tisminetzky, S. G., Glišin, V., & Ljubijankić, G. (2003). Expression and characterization of human interferon-β1 in the methylotrophic yeast Pichia pastoris. Biotechnology and Applied Biochemistry, 38, 257–265.
Gao, Z., Bai, G., Chen, J., Zhang, Q., Pan, P., Bai, F., & Geng, P. (2009). Development, characterization, and evaluation of a fusion protein of a novel glucagon-like peptide-1 (GLP-1) analog and human serum albumin in Pichia pastoris. Bioscience, Biotechnology, and Biochemistry, 73, 688–694.
Hou, J., Yan, R., Yang, L., Wu, Z., Wang, C., Ding, D., Li, N., Ma, C., & Li, M. (2007). High-level expression of fusion protein containing 10 tandem repeated GLP-1 analogs in yeast Pichia pastoris and its biological activity in a diabetic rat model. Bioscience, Biotechnology, and Biochemistry, 71, 1462–1469.
Macauley-Patrick, S., Fazenda, M. L., McNeil, B., & Harvey, L. M. (2005). Heterologous protein production using the Pichia pastoris expression system. Yeast, 22, 249–270.
Acknowledgments
The authors thank the National Natural Science Foundation of China (No.31171658) for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, P., Zhu, Q., Chen, K. et al. Improving the Secretory Production of the Heterologous Protein in Pichia pastoris by Focusing on Protein Folding. Appl Biochem Biotechnol 175, 535–548 (2015). https://doi.org/10.1007/s12010-014-1292-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1292-5