Abstract
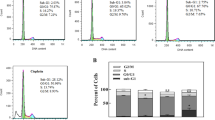
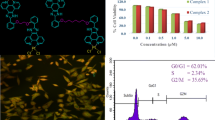
The two six-coordinate Pt(IV) complexes, containing bidentate nitrogen donor/methyl ligands with general formula [Pt(X)2Me2(tbu2bpy)], where tbu2bpy = 4,4′-ditert-butyl-2,2′-bipyridine and X = Cl (C1) or Br (C2), serving as the leaving groups were synthesized for evaluation of their anticancer activities and DNA binding properties. To examine anticancer activities of the synthetic complexes, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and ethidium bromide/acridine orange (EB/AO) staining method were performed. The binding properties of these complexes to DNA and purine nucleotides were examined, using different spectroscopic techniques. These complexes demonstrated significant anticancer activities against three cancer cell lines Jurkat, K562, and MCF-7. On the basis of the results of EB/AO staining, C1 and C2 were also capable to induce apoptosis in cancer cells. These complexes comprise halide leaving groups, displaying different departure rates; accordingly, they demonstrated slightly dissimilar anticancer activity and significantly different DNA/purine nucleotide binding properties. The results of DNA interaction studies of these complexes suggest a mixed-binding mode, comprising partial intercalation and groove binding. Overall, the results presented herein indicate that the newly synthesized Pt(IV) complexes are promising class of the potential anticancer agents which can be considered as molecular templates in designing novel platinum anticancer drugs. This study also highlights the importance of leaving group in anticancer activity and DNA binding properties of Pt(IV) complexes.







Similar content being viewed by others
References
Kostova, I. (2006). Platinum complexes as anticancer agents. Recent Patents on Anti-Cancer Drug Discovery, 1, 1–22.
Rozencweig, M., von Hoff, D. D., Slavik, M., & Muggia, F. M. (1977). Cis-diamminedichloroplatinum (II). A new anticancer drug. Annals of Internal Medicine, 86, 803–812.
Loh, S. Y., Mistry, P., Kelland, L. R., Abel, G., & Harrap, K. R. (1992). Reduced drug accumulation as a major mechanism of acquired resistance to cisplatin in a human ovarian carcinoma cell line: circumvention studies using novel platinum (II) and (IV) ammine/amine complexes. British Journal of Cancer, 66, 1109–1115.
Whiteside, M. A., Piyathilake, C. J., Bushell, T. M., & Johanning, G. L. (2006). Intrinsic cisplatin resistance in lung and ovarian cancer cells propagating in medium acutely depleted of folate. Nutrition and Cancer, 54, 274–284.
Kartalou, M., & Essigmann, J. M. (2001). Mechanisms of resistance to cisplatin. Mutation Research, 478, 23–43.
Florea, A. M., & Büsselberg, D. (2011). Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers., 3, 1351–1371.
Fuertes, M. A., Alonso, C., & Pérez, J. M. (2003). Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chemical Reviews, 103, 645–662.
Wilson, J. J., & Lippard, S. J. (2011). Synthesis, characterization, and cytotoxicity of platinum(IV) carbamate complexes. Inorganic Chemistry, 50, 3103–3115.
Galanski, M., & Keppler, B. K. (2007). Searching for the magic bullet: anticancer platinum drugs which can be accumulated or activated in the tumor tissue. Anti-Cancer Agents in Med Chem., 7, 55–73.
Hill, G. S., Vittal, J. J., & Puddephatt, R. J. (1997). Methyl (hydrido) platinum (IV) complexes: X-ray structure of the first (μ-Hydrido) diplatinum (IV) complex. Organometallics, 16, 1209–1217.
Kelly, M. E., Gómez-Ruiz, S., Kluge, R., Merzweiler, K., Steinborn, D., Wagner, C., et al. (2009). Studies of mononuclear and dinuclear complexes of dibromodimethylplatinum (IV): preparation, characterization and crystal structures. Inorganica Chimica Acta, 362, 1323–1332.
Yousefi, R., Aghevlian, S., Mokhtari, F., Samouei, H., Rashidi, M., Nabavizadeh, S. M., et al. (2012). The anticancer activity and HSA binding properties of the structurally related platinum (II) complexes. Applied Biochemistry and Biotechnology, 167, 861–872.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55–63.
Tabassum, S., Khan, R. A., Arjmand, F., Juvekar, A. S., & Zingde, S. M. (2010). Synthesis of carbohydrate-conjugate heterobimetallic Cu(II)-Sn(2)(IV) and Zn(II)-Sn(2)(IV) complexes; their interactions with CT DNA and nucleotides; DNA cleavage, in-vitro cytotoxicity. European Journal of Medicinal Chemistry, 45, 4797–8067.
Bednarski, P. J., Mackay, F. S., & Sadler, P. J. (2007). Photoactivatable platinum complexes. Anti-Cancer Agents in Medicinal Chemistry, 7, 75–93.
Abu-Surrah, A. S., & Kettunen, M. (2006). Platinum group antitumor chemistry: design and development of new anticancer drugs complementary to cisplatin. Current Medicinal Chemistry, 13, 1337–1357.
Isse, A. A., Gennaro, A., Lin, C. Y., Hodgson, J. L., Coote, M. L., & Guliashvili, T. (2011). Mechanism of carbon–halogen bond reductive cleavage in activated alkyl halide initiators relevant to living radical polymerization: theoretical and experimental study. Journal of the American Chemical Society, 133, 6254–6264.
Kasibhatla, S., Amarante-Mendes, G. P., Finucane, D., Brunner, T., Bossy-Wetzel, E. and Green, D. R. (2006). Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSH Protoc. doi:10.1101/pdb.prot4493.
Xu, X., Gao, X., Jin, L., Bhadury, P. S., Yuan, K., Hu, D., et al. (2011). Antiproliferation and cell apoptosis inducing bioactivities of constituents from Dysosma versipellis in PC3 and Bcap-37 cell lines. Cell Division, 6, 14–26.
Barton, J. K., Danishefsky, A. T., & Goldberg, J. M. (1984). Tris(phenanthroline) ruthenium (II): stereoselectivity in binding to DNA. Journal of the American Chemical Society, 106, 2172–2176.
Haas, K. L., & Franz, K. J. (2009). Application of metal coordination chemistry to explore and manipulate cell biology. Chemical Reviews, 109, 4921–4960.
Turel, I., & Kljun, J. (2011). Interactions of metal ions with DNA, its constituents and derivatives, which may be relevant for anticancer research. Current Topics in Medicinal Chemistry, 11, 2661–2687.
Waring, M. J. (1965). Complex formation between ethidium bromide and nucleic acids. Journal of Molecular Biology, 13, 269–282.
LePecq, J. B., & Paoletti, C. (1967). A fluorescent complex between ethidium bromide and nucleic acids. Physical–chemical characterization. Journal of Molecular Biology, 27, 87–106.
Uma Maheswari, P., & Palaniandavar, M. (2004). DNA binding and cleavage properties of certain tetrammine ruthenium(II) complexes of modified 1,10-phenanthrolines—effect of hydrogen-bonding on DNA-binding affinity. Journal of Inorganic Biochemistry, 98, 219–230.
Tarushi, A., Christofis, P., & Psomas, G. (2007). Synthesis, characterization and interaction with DNA of mononuclear metal complexes with oxolinic acid. Polyhedron, 26, 3963–3972.
Acknowledgments
We would like to acknowledge the financial supports of research council of Shiraz University for this study. Also the supports of Iran National Science Foundation (grant nos. 92001695 and 90005876) are acknowledged.
Conflict of Interest
The authors declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 310 kb)
Rights and permissions
About this article
Cite this article
Pouryasin, Z., Yousefi, R., Nabavizadeh, S.M. et al. Anticancer and DNA Binding Activities of Platinum (IV) Complexes; Importance of Leaving Group Departure Rate. Appl Biochem Biotechnol 172, 2604–2617 (2014). https://doi.org/10.1007/s12010-013-0700-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0700-6