Abstract
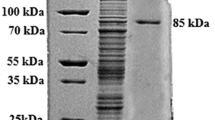
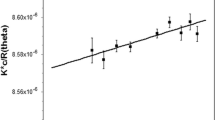
The kinetic characteristics of two Rhizopus oryzae exo-polygalacturonases acting on galacturonic acid oligomers (GalpA) were determined using isothermal titration calorimetry (ITC). RPG15 hydrolyzing (GalpA)2 demonstrated a K m of 55 μM and k cat of 10.3 s−1 while RPG16 was shown to have greater affinity for (GalpA)2 with a K m of 16 μM, but lesser catalytic activity with a k cat of 3.9 s−1. Both enzymes were inhibited by the product, galacturonic acid, with app K i values of 886 and 501 μM for RPG15 and RPG16, respectively. RPG15 exhibited greater affinity for (GalpA)3 with a K m of 9.2 μM and a similar k cat at 10.7 s−1 relative to (GalpA)2. Catalytic constants for RPG16 hydrolyzing (GalpA)3 could not be determined; however, single-injection ITC assays suggest a distinct preference and catalytic rate for (GalpA)3 relative to (GalpA)2. Thermodynamic parameters of a series of galacturonic acid oligomers binding to RPG15 were determined and exhibited some distinct differences from RPG16 binding thermodynamics, providing potential clues to the differing kinetic characteristics of the two exo-polygalacturonase enzymes.



Similar content being viewed by others
References
Niture, S. K. (2008). Biologia, 63, 1–19.
Markovič, O., & Janaček, Š. (2001). Protein Engineering, 14, 615–631.
Henrissat, B., & Davies, G. (1997). Current Opinion in Structural Biology, 7, 637–644.
Zhang, J., Henriksson, H., Szabo, I. J., Henriksson, G., & Johansson, G. (2005). Journal of Industrial Microbiology and Biotechnology, 32, 431–438.
Stotz, H. U., Bishop, J. G., Bergmann, C. W., Koch, M., Albersheim, P., Darvill, A. G., et al. (2000). Physiological and Molecular Plant Pathology, 56, 117–130.
Kars, I., Krooshof, G. H., Wagemakers, L., Joosten, R., Benen, J. A. E., & van Kan, J. A. L. (2005). The Plant Journal, 43, 213–225.
Kester, H. C. M., & Visser, J. (1990). Biotechnology and Applied Biochemistry, 12, 150–160.
Kluskens, L. D., van Alebeek, G. W. M., Walther, J., Voragen, A. G. H., de Vos, W. M., & van der Oost, J. (2005). FEBS Journal, 272, 5464–5473.
Kester, H. C. M., Kusters-Van Someren, M. A., Müller, Y., & Visser, J. (1996). European Journal of Biochemistry, 240, 738–746.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428.
Somogyi, M. (1952). Journal of Biological Chemistry, 153, 375–380.
Breuil, C., & Saddler, J. N. (1985). Enzyme and Microbial Technology, 7, 327–332.
Baumann, M. J., Murphy, L., Lei, N., Krogh, K., Borch, K., & Westh, P. (2011). Analytical Biochemistry, 410, 19–26.
Mertens, J. A., & Bowman, M. J. (2011). Current Microbiology, 62, 1173–1178.
Jordan, D. B., Mertens, J. A., & Braker, J. D. (2009). Biochimica et Biophysica Acta, 1794, 144–158.
Krokeide, I., Synstad, B., Gåseidnes, S., Horn, S. J., Eijsink, V. G. H., & Sørlie, M. (2007). Analytical Biochemistry, 363, 128–134.
Dennhart, N., Fukamizo, T., Brzezinski, R., Lacombe-Harvey, M., & Letzel, T. (2008). Journal of Biotechnology, 134, 253–260.
Todd, M. J., & Gomez, J. (2001). Analytical Biochemistry, 296, 179–187.
Krokeide, I., Eijsink, V. G. H., & Sørlie, M. (2007). Thermochim. Acta, 454, 144–146.
Karim, N., & Kidokoro, S. (2004). Thermochim. Acta, 412, 91–96.
Jeoh, T., Baker, J. O., Ali, M. K., Himmel, M. E., & Adney, W. S. (2005). Analytical Biochemistry, 347, 244–253.
Bohlin, C., Olsen, S. N., Morant, M. D., Patkar, S., Borch, K., & Westh, P. (2010). Biotechnology and Bioengineering, 107, 943–952.
Mertens, J. A., Hector, R. E., & Bowman, M. J. (2012). Thermochim. Acta, 527, 219–222.
Gill, S. C., & von Hippel, P. H. (1989). Analytical Biochemistry, 182, 319–326.
Leatherbarrow, R. J. (2001). Grafit version 5. Horley: Erithacus Software Ltd.
Cho, S. W., Lee, S., & Shin, W. (2001). Journal of Molecular Biology, 314, 863–878.
van Santen, Y., Nenen, J. A. E., Schröter, K., Kalk, K. H., Armand, S., Visser, J., et al. (1999). Journal of Biological Chemistry, 274, 30474–30480.
Norberg, A. L., Karlsen, V., Hoell, I. A., Bakke, I., Eijsink, V. G. H., & Sørlie, M. (2010). FEBS Letters, 585, 4581–4585.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Mertens, J.A. Kinetic Properties of Two Rhizopus Exo-polygalacturonase Enzymes Hydrolyzing Galacturonic Acid Oligomers Using Isothermal Titration Calorimetry. Appl Biochem Biotechnol 170, 2009–2020 (2013). https://doi.org/10.1007/s12010-013-0336-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0336-6