Abstract
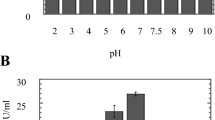
Among all endophytic keratinolytic fungal isolates recovered from marine soft coral Dendronephthya hemprichii, Penicillium spp. Morsy1 was selected as the hyperactive keratinolytic strain under solid substrate fermentation of different agriculture and poultry wastes. The optimization of extraction process, physicochemical parameters affecting the keratinase production in solid-state fermentation, and the purified keratinase parameters were studied. Maximum keratinase activity (1,600 U g−1, initial dry substrate) was recovered from moldy bran with 0.1% Tween 80. The optimized production conditions were rice straw as carbon source, pH of medium 6, growth temperature 26 °C, initial moisture content of 80% (v/w), inoculum size of 105 spores ml−1, and an average particle size of the substrate 0.6 mm (3,560 U g−1, initial dry substrate after 5 days of fermentation). Two types of keratinase (Ahm1 and Ahm2) were purified from the culture supernatant through ammonium sulfate precipitation, DEAE-Sepharose, and gel filtration chromatography. Enzyme molecular weights were 19 kDa (Ahm1) and 40 kDa (Ahm2). The kinetic parameters of purified keratinases were optimized for the hydrolysis of azokeratin by Ahm1 (pH 7.0–8.0, stable in pH range of 6.0 to 8.0 at 50 °C) and Ahm2 enzymes (pH 10.0–11.0, stable in pH range of 6.0 to 11.0 at 60–65 °C). Whereas inhibitors of serine (phenylmethylsulfonyl fluoride) and cysteine (iodoacetamide) proteases had minor effects on both Ahm1 and Ahm2 activity, both keratinases were strongly inhibited by chelating agents EDTA and EGTA. These findings suggest that serine and cysteine residues are not involved in the catalytic mechanisms, and they are metalloproteases.








Similar content being viewed by others
References
Agrawal, D., Patidar, P., Banerjee, T., & Patil, S. (2004). Production of alkaline protease by Penicillium sp. under SSF conditions and its application to soy protein hydrolysis. Process Biochemistry, 39, 977–981.
Fernendez-Lahore, H. M., Fraile, E. R., & Cascone, O. (1998). Acid protease recovery from a solid-state fermentation system. Journal of Biotechnology, 62, 83–93.
Kaul, S., & Sumbali, G. (1997). Keratinolysis by poultry farm soil fungi. Mycopathologia, 139, 137–140.
Ikasari, L., & Mitchell, D. A. (1996). Leaching and characterization of Rhizopus oligosporus acid protease from solid state fermentation. Enzyme Microbial Technology, 19, 171–175.
Moo-Young, M., Moreira, A. R., & Tengerdy, R. P. (1983). Principles of solid-state fermentation. In J. F. Smith, D. R. Berry & B. Kristiansen (Eds.), The filamentous fungi (Vol. IV, pp. 117–144). London: Adward Arnold.
Viniegra-Gonzalez, G., Favela-Torres, E., Aguilar, C. N., Romero-Gomez, S. J., Dıaz-Godınz, G., & Augur, C. (2003). Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochemical Engineering, 13, 157–167.
Holler, U., Wright, A. D., Matthee, G. F., Konig, G. M., Draeger, S., & Aust, H. J. (2000). Fungi from marine sponges: Diversity, biological activity and secondary metabolites. Mycological Research, 104, 1354–1765.
Pitt, J. I., Samson, R. A., & Frisvad, J. C. (2000). List of accepted species and their synonyms in the family Trichocomaceae. In R. A. Samson & J. I. Pitt (Eds.), Integration of modern methods for Penicillium and Aspergillus classification. Amsterdam: Harwood Academic Publishers.
Pitt, J. I. (1979). The genus Penicillium and its teleomorphic states, Eupenicillium and Talaromyces. London: Academic.
Sorensen, J. L., Mogensen, J. M., Thrane, U., & Andersen, B. (2009). Potato carrot agar with manganese as an isolation medium for Alternaria, Epicoccum and Phoma. International Journal of Food Microbiology, 130, 22–26.
Sangali, S., & Brandelli, A. (2000). Feather keratin hydrolysis by a Vibrio sp. strain kr2. Journal of Applied Microbiology, 89, 735–743.
Suresh, P. V., & Chandrasekaran, M. (1999). Impact of process parameters on chitinase production by an alkalophilic marine Beauveria bassiana in solid state fermentation. Process Biochemistry, 34, 257–267.
Layne, E. (1957). Spectrophotometic and turbidimetric methods for measuring proteins. Method in Enzymology, 3, 447–455.
Stoschek, C. M. (1990). Quantitation of protein. Method in Enzymology, 182, 50–69.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Priest, F., & Austin, B. (1992). In F. Preist & B. Austin (Eds.), Modern bacterial taxonomy. Edinburgh, UK: Henot-Watt University Press.
Dozie, I. N. S., Okeke, C. N., & Unaeze, N. C. (1994). A thermostable, alkaline-active, keratinolytic proteinase from Chrysosporium keratinophilum. World Journal of Microbiology and Biotechnology, 10, 563–567.
Williams, M., & Shih, J. C. H. (1989). Enumeration of some microbial groups in thermophilic poultry waste digesters and enrichment of a feather degrading culture. Journal of Applied Bacteriology, 67, 25–35.
Lin, X., Lee, C. G., Casale, E. S., & Shih, J. C. H. (1992). Purification and characterization of a keratinase from a feather-degrading Bacillus licheniformis strain. Applied and Environmental Microbiology, 58, 3271–3275.
Tunga, R., Banerjee, R., & Bhattacharyya, B. C. (1999). Some studies on optimization of extraction process for protease production in SSF. Bioprocess Engineering, 20, 485–489.
Cruz, D., Hidalgo-Gallego, A., Lora, J. M., Benitez, T., Pintor-Toro, J. A., & Llobell, A. (1992). Isolation and characterization of three chitinases from Trichoderma harzianum. European Journal of Biochemistry, 206, 859–867.
Cai, C.-G., Lou, B.-G., & Zheng, X.-D. (2008). Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. Journal of Zhejiang University Science, 1, 60–67.
Ramesh, M. V., & Lonsane, B. K. (1990). Critical importance of moisture content of the medium in alpha amylase production by Bacillus licheniformis M 27 in a solid state fermentation system. Applied Microbiology and Biotechnology, 33, 501–505.
Sonia, K. G., Chadha, B. S., & Saini, H. S. (2005). Sorghum straw for xylanase hyper-production by Thermomyces lanuginosus (D2W3) under solid-state fermentation. Bioresource Technology, 96, 1561–1569.
Botella, C., Diaz, A., Ory, I., Webb, C., & Blandino, A. (2007). Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochemistry, 42, 98–101.
Rajgopalan, S., & Modak, J. M. (1995). Modelling of heat and mass transfer for solid-state fermentation process in trey bioreactor. Bioprocess Biosystem Engineering, 13, 161–169.
Karadzic, I., Masui, A., & Fujiwara, N. (2004). Purification and characterization of a protease from Pseudomonas aeruginosa grown in cutting oil. Journal of Bioscience and Bioengineering, 98, 145–152.
Nakasone, N., Toma, C., Song, T., & Iwanaga, M. (2004). Purification and characterization of a novel metalloprotease isolated from Aeromonas caviae. FEMS Microbiology Letters, 237, 127–132.
Kim, S. S., Kim, Y. J., & Rhee, I. K. (2001). Purification and characterization of a novel extracellular protease from Bacillus cereus KCTC 3674. Archives of Microbiology, 175, 458–461.
Hutadilok-Towatana, A., Paindpong, A., & Suntinanalert, P. (1999). Purification and characterization of an extracellular protease from alkaliphilic and thermophilic Bacillus sp. PS719. Journal of Bioscience and Bioengineering, 87, 581–587.
Oliveira, L. A., Porto, A. L. F., & Tambourgi, E. B. (2006). Production of xylanase and protease by Penicillium janthinellum CRC 87M–115 from different agricultural wastes. Bioresuorce Technology, 97, 862–867.
Allpress, J. D., Mountain, G., & Gowland, P. C. (2002). Production, purification, and characterization of an extracellular keratinase from Lysobacter NCIMB 9497. Letters in Applied Microbiology, 34, 337–342.
Farag, A. M., & Hassan, M. A. (2004). Purification, characterization and immobilization of a keratinase from Aspergillus orizae. Enzyme and Microb Technology, 34, 85–93.
Venter, H., Osthoff, G., & Litthauer, D. (1999). Purification and characterization of a metalloprotease from Chryseobacterium indologenes Ix9a and determination of the amino acid specificity with electrospray spectrometry. Protein Expression and Purification, 15, 282–295.
Kumar, C. G., & Takagi, H. (1999). Microbial alkaline proteases: From a bioindustrial viewpoint. Biotechnology Advances, 17, 561–594.
Secades, P., & Guijarro, J. A. (2001). Purification and characterization of a psychrophilic calcium-induced growth-phase-dependent metalloprotease from the fish pathogen Flavobacterium psychrophilum. Applied and Environmental Microbiology, 67, 2436–2444.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Gendy, M.M.A. Keratinase Production by Endophytic Penicillium spp. Morsy1 Under Solid-State Fermentation Using Rice Straw. Appl Biochem Biotechnol 162, 780–794 (2010). https://doi.org/10.1007/s12010-009-8802-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8802-x