Abstract
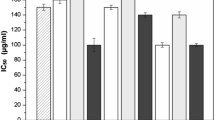
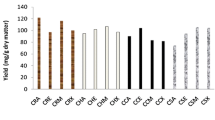
The extraction of antioxidant phenolic compounds from coffee silverskin (CS) was studied. Firstly, the effect of different solvents (methanol, ethanol, acetone, and distilled water) on the production of antioxidant extracts was evaluated. All the extracts showed antioxidant activity (FRAP and DPPH assays), but those obtained with methanol and ethanol had significantly higher (p < 0.05) DPPH inhibition than the remaining ones. Due to the lower toxicity, ethanol was selected as extraction solvent, and further experiments were performed in order to define the solvent concentration, solvent/solid ratio, and time to maximize the extraction results. The best condition to produce an extract with high content of phenolic compounds (13 mg gallic acid equivalents/g CS) and antioxidant activity [DPPH = 18.24 μmol Trolox equivalents/g CS and FRAP = 0.83 mmol Fe(II)/g CS] was achieved when using 60 % ethanol in a ratio of 35 ml/g CS, during 30 min at 60–65 °C.




Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- CS:
-
Coffee silverskin
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- FRAP:
-
Ferric reducing antioxidant power
- GAE:
-
Gallic acid equivalents
- PC:
-
Phenolic compounds
- QE:
-
Quercetin
- TE:
-
Trolox equivalents
References
Alothman, M., Bhat, R., & Karim, A. A. (2009). Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chemistry, 115(3), 785–788.
Ao, C., Higa, T., Khanh, T. D., Upadhyay, A., & Tawata, S. (2011). Antioxidant phenolic compounds from Smilax sebeana Miq. LWT- Food Science and Technology, 44(7), 1681–1686.
Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76.
Borrelli, R. C., Esposito, F., Napolitano, A., Ritieni, A., & Fogliano, V. (2004). Characterization of a new potential functional ingredient: Coffee silverskin. Journal of Agricultural and Food Chemistry, 52(5), 1338–1343.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein using the principles of protein-dye binding. Analytical Biochemistry, 72, 248–255.
Chang, C.-C., Yang, M.-H., Wen, H.-M., & Chern, J.-C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10(3), 178–182.
Chirinos, R., Rogez, H., Campos, D., Pedreschi, R., & Larondelle, Y. (2007). Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Separation and Purification Technology, 55(2), 217–225.
Cortazar, E., Bartolomé, L., Delgado, A., Etxebarria, N., Fernández, L. A., Usobiaga, A., & Zuloaga, O. (2005). Optimisation of microwave-assisted extraction for the determination of nonylphenols and phthalate esters in sediment samples and comparison with pressurised solvent extraction. Analytica Chimica Acta, 534(2), 247–254.
Dorta, E., Lobo, M. G., & González, M. (2012). Reutilization of mango by-products: Study of the effect of extraction solvent and temperature on their antioxidant properties. Journal of Food Science, 77, C80–C88.
Gan, C.-Y., & Latiff, A. A. (2011). Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chemistry, 124(3), 1277–1283.
Hidalgo, M., Sánchez-Moreno, C., & de Pascual-Teresa, S. (2010). Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chemistry, 121(3), 691–696.
Jiménez, J. P., Serrano, J., Tabernero, M., Arranz, S., Díaz-Rubio, M. E., García-Diz, L., Goñi, I., & Saura-Calixto, F. (2008). Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors. Nutrition, 24(7–8), 646–653.
Karadag, A., Ozcelik, B., & Saner, S. (2009). Review of methods to determine antioxidant capacities. Food Analytical Methods, 2(1), 41–60.
Kim, D.-O., & Lee, C. Y. (2002). Extraction and isolation of polyphenolics. In R. E. Wrolstad (Ed.), Current protocols in food analytical chemistry (pp. I1.2.1–I1.2.12). New York: Wiley.
Lafka, T. I., Sinanoglou, V., & Lazos, E. S. (2007). On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chemistry, 104(3), 1206–1214.
Lebovka, N., Vorobiev, N., & Chemat, E. (2011). Enhancing extraction processes in the food industry. Boca Raton: CRC Press.
Liu, F. F., Ang, C. Y. W., & Springer, D. (2000). Optimization of extraction conditions for active components in Hypericum perforatum using response surface methodology. Journal of Agricultural and Food Chemistry, 48(8), 3364–3371.
Machado, E. M. S., Rodriguez-Jasso, R. M., Teixeira, J. A., & Mussatto, S. I. (2011). Growth of fungal strains on coffee industry residues with removal of polyphenolic compounds. Biochemical Engineering, 60, 87–90.
Markom, M., Hasan, M., Daud, W. R. W., Singh, H., & Jahim, J. M. (2007). Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Separation and Purification Technology, 52(3), 487–496.
Martins, S., Aguilar, C. N., Garza–Rodriguez, I., Mussatto, S. I., & Teixeira, J. A. (2010). Kinetic study of nordihydroguaiaretic acid recovery from Larrea tridentata by microwave–assisted extraction. Journal of Chemical Technology and Biotechnology, 85(8), 1142–1147.
Martins, S., Mussatto, S. I., Martínez-Avila, G., Montañez-Saenz, J., Aguilar, C. N., & Teixeira, J. A. (2011). Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnology Advances, 29, 365–373.
Meneses, N. G. T., Martins, S., Teixeira, J. A., & Mussatto, S. I. (2013). Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Separation and Purification Technology, 108, 152–158.
Murthy, P. S., & Naidu, M. M. (2012). Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food and Bioprocess Technology, 5(3), 897–903.
Mussatto, S. I., Machado, E. M. S., Martins, S., & Teixeira, J. A. (2011a). Production, composition, and application of coffee and its industrial residues. Food and Bioprocess Technology, 4(5), 661–672.
Mussatto, S. I., Ballesteros, L. F., Martins, S., & Teixeira, J. A. (2011b). Extraction of antioxidant phenolic compounds from spent coffee grounds. Separation and Purification Technology, 83, 173–179.
Narita, Y., & Inouye, K. (2012). High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chemistry, 135(3), 943–949.
Pompeu, D. R., Silva, E. M., & Rogez, H. (2009). Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using response surface methodology. Bioresource Technology, 100(23), 6076–6082.
Prasad, K. N., Yang, E., Yi, C., Zhao, M., & Jiang, Y. (2009). Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innovative Food Science & Emerging Technologies, 10(2), 155–159.
Prasad, K. N., Hassan, F. A., Yang, B., Kong, K. W., Ramanan, R. N., Azlan, A., & Ismail, A. (2011). Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm. peels. Food Chemistry, 128(4), 1121–1127.
Rodríguez-Meizoso, I., Jaime, L., Santoyo, S., Señoráns, F., Cifuentes, A., & Ibáñez, E. (2010). Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. Journal of Pharmaceutical and Biomedical Analysis, 51(2), 456–463.
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158.
Spigno, G., Tramelli, L., & De Faveri, D. M. (2007). Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. Journal of Food Engineering, 81(1), 200–208.
Stratil, P., Klejdus, B., & Kubáň, V. (2007). Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta, 71(4), 1741–1751.
Trabelsi, N., Megdiche, W., Ksouri, R., Falleh, H., Oueslati, S., Soumaya, B., Hajlaoui, H., & Abdelly, C. (2010). Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT- Food Science and Technology, 43(4), 632–639.
Wang, S. Y., & Lin, H.-S. (2000). Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. Journal of Agricultural and Food Chemistry, 48(2), 140–146.
Wijekoon, M. M. J. O., Bhat, R., & Karim, A. A. (2011). Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. Journal of Food Composition and Analysis, 24(4), 615–619.
Acknowledgments
This work was supported by the Portuguese Foundation for Science and Technology (FCT). The authors gratefully acknowledge Teresa Conde, student of Biological Engineering, for the help and interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ballesteros, L.F., Teixeira, J.A. & Mussatto, S.I. Selection of the Solvent and Extraction Conditions for Maximum Recovery of Antioxidant Phenolic Compounds from Coffee Silverskin. Food Bioprocess Technol 7, 1322–1332 (2014). https://doi.org/10.1007/s11947-013-1115-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1115-7