Abstract
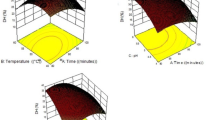
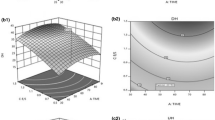
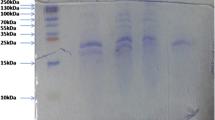
Fish protein hydrolysate was produced from the viscera of yellowfin tuna (Thunnus albacares). Hydrolysis conditions (enzyme activity, temperature, and time) were optimized using response surface methodology. A factorial design was applied to minimize enzyme utilization and modeling of degree of hydrolysis (r 2 = 0.94). Lack-of-fit test revealed a non-significant value for the model, indicating that the regression equation was adequate for predicting the degree of hydrolysis under any combination of the variables (P < 0.05). The optimum conditions to reach the highest degree of hydrolysis were: 60.4 °C, 90.25 min, and a protease (Alcalase 2.4 L) activity of 70.22 AU/kg protein. The spray-dried tuna visceral protein hydrolysates had relatively high protein (72.34%) and low lipid (1.43%) content. The chemical score of the hydrolysate indicated that it fulfils adult human nutritional requirements except for methionine. Lysine and methionine were the first and the second limiting amino acids in that order. Phenylalanine was the predominant amino acid in the hydrolysates with respect to common carp requirement. In addition, the protein efficiency ratio of tuna visceral hydrolysate was 2.85–5.35.


Similar content being viewed by others
References
Adler-Nissen, J. (1984). Control of the proteolytic reaction and of the level of bitterness in protein hydrolysis process. Journal of Chemical Technology and Biotechnology, 34B, 215–222.
Adler-Nissen, J. (1986). Enzymatic hydrolysis of food proteins (pp. 57–109). Copenhagen: Elsevier Applied Science Publishers.
Alsmeyer, R. H., Cunningham, A. E., & Happich, M. L. (1974). Equations predicting PER from amino acid analysis. Food Technology, 28(7), 34–40.
Antoine, F. R., Wei, C. I., Littell, R. C., & Marshall, M. R. (1999). HPLC method for analysis of free amino acids in fish using o-Phthaldialdehyde precolumn derivatization. Journal of Agricultural and Food Chemistry, 47, 5100–5107.
AOAC (2002). In: Hortwitz W (ed) Official Methods of Analysis of AOAC International, 17th edn. Gaithersburg: AOAC International
Aspmo, S. I., Horn, S. J., & Eijsink, V. G. H. (2005). Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochemistry, 40, 1957–1966.
Batista, I., Ramos, C., Coutinho, J., Bandarra, N. M., & Nunes, M. L. (2010). Characterization of protein hydrolysates and lipids obtained from black scabbardfish (Aphanopus carbo) by-products and antioxidative activity of the hydrolysates produced. Process Biochemistry, 45, 18–24.
Benjakul, B., & Morrissey, M. T. (1997). Protein hydrolysates from Pacific whiting solid wastes. Journal of Agricultural and Food Chemistry, 45, 3423–3430.
Bhaskar, N., & Mahendrakar, N. S. (2008). Protein hydrolysate from visceral waste proteins of Catla (Catla catla): optimization of hydrolysis conditions for a commercial neutral protease. Bioresource Technology, 99(10), 4105–4111.
Bhaskar, N., Benila, T., Radha, C., & Lalitha, R. G. (2008). Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresource Technology, 99(2), 335–343.
Cao, W., Zhang, C., Hong, P., & Ji, H. (2008). Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chemistry, 109, 176–183.
Dabrowski, K., & Guderley, H. (2002). Intermediary metabolism. In J. E. Halver & R. W. Hardy (Eds.), Fish Nutrition (3rd ed., pp. 309–367). Boston: Elsevier Science.
Diniz, A. M., & Martin, A. M. (1997). Optimization of nitrogen recovery in the enzymatic hydrolysis of dogfish (Squalus acanthias) protein: Composition of the hydrolysates. International Journal of Food Sciences and Nutrition, 48, 191–200.
FAO. (2006). Food and agricultural organisation of the United Nations. Year book of fishery statistics. Rome, 98, 1&2.
FAO/WHO (1990). Energy and protein requirements. Report of joint FAO/WHO/UNU Expert Consultation Technical Report. FAO/WHO and United Nations University, Geneva, Series No. 724, pp. 116–129.
Gbogouri, G. A., Linder, M., Fanni, J., & Parmentier, M. (2004). Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. Journal of Food Science, 69, 615–622.
Guerard, F., Guimas, L., & Binet, A. (2002). Production of tuna waste hydrolysates by a commercial neutral protease preparation. Journal of Molecular Catalysis. B, Enzymatic, 19–20, 489–498.
Hoyle, N. T., & Merritt, J. H. (1994). Quality of fish protein hydrolysate from herring (Clupea harengus). Journal of Food Science, 59, 76–79.
IFO (2006). Iranian Fisheries Organization. www.shilat.com
Kristinsson, H. G., & Rasco, B. A. (2000a). Fish protein hydrolysates: production, biochemical and functional properties. Critical Reviews in Food Science and Nutrition, 40, 43–81.
Kristinsson, H. G., & Rasco, B. A. (2000b). Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. Journal of Agricultural and Food Chemistry, 48, 657–666.
Layne, E. (1957). Spectrophotometric and turbidimetric methods for measuring proteins. Methods in Enzymology, vol. 3 (p. 450). New York: Academic Press.
Lee, Y. B., Elliot, J. G., Rickansrud, D. A., & Mugberg, E. C. (1978). Predicting protein efficiency ratio by the chemical determinations of connective tissue content in meat. Journal of Food Science, 43, 1359–1362.
Little, R. C., Feund, R. J., & Spector, P. C. (1991). SAS system for linear models (3rd ed.). Cary: SAS Institute.
Nilsang, S., Lertsiri, S., Suphantharika, M., & Assavanig, A. (2005). Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. Journal of Food Engineering, 70, 571–578.
Novozymes. (2007). Alcalase® 2.4 L FG. Product data sheet. www.novozymes.com.
NRC (1993). National Research Council. National Academy of Sciences, Nutrient Requirements of Fish (p. 124). Washington: National Academy Press.
Onodenalore, A. C., & Shahidi, F. (1996). Protein dispersions and hydrolysates from shark (Isurus oxyrinchus). Journal of Aquatic Food Product and Technology, 5, 43–59.
Ovissipour, M., & Ghomi, M. R. (2009). Biotechnology in seafood production (1st ed., pp. 1–198). Tehran: Islamic Azad University Publication.
Ovissipour, M., Abedian, A. M., Motamedzadegan, A., Rasco, B., Safari, R., & Shahiri, H. (2009a). The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from the Persian sturgeon (Acipenser persicus) viscera. Food Chemistry, 115, 238–242.
Ovissipour, M., Safari, R., Motamedzadegan, A., & Shabanpour, B. (2009b). Chemical and biochemical hydrolysis of Persian sturgeon (Acipenser persicus) visceral protein. Food and Bioprocess Technology, doi:10.1007/s11947-009-0284-x
Pigott, G., & Tucker, B. (2002). Special feeds. In J. E. Halver & R. W. Hardy (Eds.), Fish nutrition (3rd ed., pp. 651–669). Boston: Elsevier Science.
Raa, J., & Gildberg, A. (1982). Fish silage: a review. CRC Critical Reviews in Food Science and Nutrition, 16, 383–419.
Safari, R., Motamedzadegan, A., Ovissipour, M., Regenstein, J. M., Gildberg, A., & Rasco, B. (2009). Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food and Bioprocess Technology. doi:10.1107/s11947-009-0225-8.
Shahidi, F., Naczk, M., Pegg, R. B., & Synowiecki, J. (1991). Chemical composition and nutritional value of processing discards of cod (Gadus murhua). Food Chemistry, 42, 145–151.
Shahidi, F., Han, X. Q., & Syniwiecki, J. (1995). Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chemistry, 53, 285–293.
Shankar, T. J., Sokhansanj, S., Bandyopadhyay, S., & Bawa, A. S. (2008). A case study on optimization of biomass flow during single-screw extrusion cooking using genetic algorithm (GA) and response surface methodology (RSM). Food and Bioprocess Technology. doi:10.1007/s11947-008-0172-9.
Šližyte, R., Rustad, T., & Storrø, I. (2005a). Enzymatic hydrolysis cod (Gadus morhua) by-products, optimization of yield and properties of lipid and protein fractions. Process Biochemistry, 40, 3680–3692.
Šližyte, R., Daukšas, E., Falch, E., Storrø, I., & Rustad, T. (2005b). Characteristics of protein fractions generated from cod (Gadus morhua) by-products. Process Biochemistry, 40, 2021–2033.
Souissi, N., Bougatef, A., Triki-Ellouz, Y., & Nasri, M. (2007). Biochemical and functional properties of Sardinella (Sardinella aurita) by-product hydrolysates. Food Technology and Biotechnology, 45, 187–194.
Walton, M. J., Cowey, C. B., Coloso, R. M., & Adron, J. W. (1986). Dietary requirements of rainbow trout for tryptophan, lysine and arginine determined by growth and biochemical measurements. Fish Physiology and Biochemistry, 2, 161–169.
Wasswa, J., Tang, J., Gu, X. H., & Yuan, X. Q. (2007). Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chemistry, 104, 1698–1704.
Acknowledgments
We express our thanks to the Ministry of Science and Tarbiat Modares University (TMU, Iran) for financial and technical supports. We would like to appreciate Prof. Turid Rustad, Prof. Barbara Rasco, Prof. David W. Levine and Mr. Ali Taheri for their scientific supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ovissipour, M., Abedian Kenari, A., Motamedzadegan, A. et al. Optimization of Enzymatic Hydrolysis of Visceral Waste Proteins of Yellowfin Tuna (Thunnus albacares). Food Bioprocess Technol 5, 696–705 (2012). https://doi.org/10.1007/s11947-010-0357-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0357-x