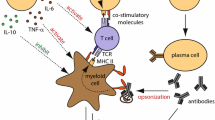
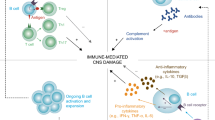
Opinion statement
The aims of this article are to discuss the potential role of B lymphocytes in the pathogenesis of multiple sclerosis (MS) and in the mechanisms of action of approved and emerging disease modifying therapies. Over the last few years, significant progress has been made in the introduction of novel pharmacologic treatments that reduce the frequency of clinical exacerbations and radiological lesion formation in relapsing remitting MS. The mechanisms of action of a number of these disease modifying therapies (DMT) implicate B cells in the pathogenesis, as well as in the regulation, of MS. Further research into B-cell subset trafficking patterns, functional activities and interactions with other immune cells in the context of neuroinflammation is likely to inform the development of future generations of DMT.
Similar content being viewed by others
Abbreviations
- MS:
-
multiple sclerosis
- DMT:
-
disease modifying therapies
- CNS:
-
central nervous system
- CSF:
-
cerebrospinal fluid
- RR:
-
relapsing-remitting
- SP:
-
secondary-progressive
- PP:
-
primary progressive
- OCBs:
-
oligoclonal bands
- IL:
-
interleukin
- EAE:
-
experimental autoimmune encephalomyelitis
- Ig:
-
immunoglobulin
- TACI:
-
Transmembrane Activator and CAML [calcium-modulator and cyclophilin ligand]-Interactor
- BLys:
-
B Lymphocyte stimulator
- APRIL:
-
a proliferation inducing ligand
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dobson R, Ramagopalan S, Davis A, Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis, and effect of latitude. J Neurol Neurosurg Psychiatry. 2013. doi:10.1136/jnnp-2012-304695.
Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–74.
Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associated with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–104.
Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–92.
O'Connor KC, Lopez-Amaya C, Gagne D, Lovato L, Moore-Odom NH, Kennedy J, et al. Anti-myelin antibodies modulate clinical expression of childhood multiple sclerosis. J Neuroimmunol. 2010;223:92–9.
Rostasy K, Mader S, Schanda K, Huppke P, Gärtner J, Kraus V, et al. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol. 2012;69:752–6.
Vogt MH, Teunissen CE, Iacobaeus E, Heijnen DA, Breij EC, Olsson T, et al. Cerebrospinal fluid anti-myelin antibodies are related to magnetic resonance measures of disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:1110–5.
Tomassini V, De Giglio L, Reindl M, Russo P, Pestalozza I, Pantano P, et al. Anti-myelin antibodies predict the clinical outcome after a first episode suggestive of MS. Mult Scler. 2007;13:1086–94.
Villar LM, Sádaba MC, Roldán E, Masjuan J, González-Porqué P, Villarrubia N, et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest. 2005;115:187–94.
Wang H, Munger KL, Reindl M, O'Reilly EJ, Levin LI, Berger T, et al. Myelin oligodendrocyte glycoprotein antibodies and multiple sclerosis in healthy young adults. Neurology. 2008;71:1142–6.
Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–72.
Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367:115–23. In this study the authors found that antibodies specific for a potassium channel expressed by glial cells are selectively elevated in the sera of individuals with MS. These results provide further evidence of autoreactive B cell dysregulation in MS and could lead to the discovery of a novel diagnostic biomarker.
McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–8.
Wolf SD, Dittel BN, Hardardottir F, Janeway CA. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–8.
Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–30.
Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–52. This paper describes a subset of B cells that produce IL-10 and suppress experimental autoimmune encephalomyelitis. The presence of analogous B cell subsets in patients with MS could explain the mechanism of action of disease modifying therapies that target B cells in MS.
Niino M, Fukazawa T, Minami N, Amino I, Tashiro J, Fujiki N, et al. CD5-positive B cell subsets in secondary progressive multiple sclerosis. Neurosci Lett. 2012;523:56–61.
Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187:537–46.
Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–56.
Byrnes AA, McArthur JC, Karp CL. Interferon-beta therapy for multiple sclerosis induces reciprocal changes in interleukin-12 and interleukin-10 production. Ann Neurol. 2002;51:165–74.
Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, et al. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naïve/memory Breg ratio during a relapse but not in remission. J Neuroimmunol. 2011;239:80–6.
Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–9.
Wright BR, Warrington AE, Edberg DD, Edberg DE, Rodriguez M. Cellular mechanisms of central nervous system repair by natural autoreactive monoclonal antibodies. Arch Neurol. 2009;66:1456–9.
Warrington AE, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri SV, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci U S A. 2000;97:6820–5.
van Engelen BG, Miller DJ, Pavelko KD, Hommes OR, Rodriguez M. Promotion of remyelination by polyclonal immunoglobulin in Theiler's virus-induced demyelination and in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1994;57(Suppl):65–8.
Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:655–61.
Rudick RA, Goelz SE. Beta-interferon for multiple sclerosis. Exp Cell Res. 2011;317:1301–11.
Ramgolam VS, Sha Y, Marcus KL, Choudhary N, Troiani L, Chopra M, et al. B cells as a therapeutic target for IFN-β in relapsing-remitting multiple sclerosis. J Immunol. 2011;186:4518–26. The authors report that B cells from untreated MS patients lose Th17 polarizing properties following incubation with recombinant IFNb in vitro.
Comabella M, Kakalacheva K, Río J, Münz C, Montalban X, Lünemann JD. EBV-specific immune responses in patients with multiple sclerosis responding to IFNβ therapy. Mult Scler. 2012;18:605–9.
Johnson KP. Glatiramer acetate for treatment of relapsing-remitting multiple sclerosis. Expert Rev Neurother. 2012;12:371–84.
Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–76.
Aharoni R, Teitelbaum D, Sela M, Arnon R. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1997;94:10821–6.
Chen M, Gran B, Costello K, Johnson K, Martin R, Dhib-Jalbut S. Glatiramer acetate induces a Th2-biased response and crossreactivity with myelin basic protein in patients with MS. Mult Scler. 2001;7:209–19.
Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, Lee L, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–43.
Carpintero R, Brandt KJ, Gruaz L, Molnarfi N, Lalive PH, Burger D. Glatiramer acetate triggers PI3Kδ/Akt and MEK/ERK pathways to induce IL-1 receptor antagonist in human monocytes. Proc Natl Acad Sci U S A. 2010;107:17692–7.
Kala M, Rhodes SN, Piao WH, Shi FD, Campagnolo DI, Vollmer TL. B cells from glatiramer acetate-treated mice suppress experimental autoimmune encephalomyelitis. Exp Neurol. 2010;221:136–45.
Begum-Haque S, Christy M, Ochoa-Reparaz J, Nowak EC, Mielcarz D, Haque A, et al. Augmentation of regulatory B cell activity in experimental allergic encephalomyelitis by glatiramer acetate. J Neuroimmunol. 2011;232:136–44.
Ireland SJ, Blazek M, Harp CT, Greenberg B, Frohman EM, Davis LS, et al. Antibody-independent B cell effector functions in relapsing remitting multiple sclerosis: clues to increased inflammatory and reduced regulatory B cell capacity. Autoimmunity. 2012;45:400–14.
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
Postigo AA, Pulido R, Campanero MR, Acevedo A, García-Pardo A, Corbi AL, et al. Differential expression of VLA-4 integrin by resident and peripheral blood B lymphocytes. Acquisition of functionally active alpha 4 beta 1-fibronectin receptors upon B cell activation. Eur J Immunol. 1991;21:2437–45.
Kowarik MC, Pellkofer HL, Cepok S, Korn T, Kümpfel T, Buck D, et al. Differential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology. 2011;76:1214–21.
Stüve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–7.
Stüve O. The effects of natalizumab on the innate and adaptive immune system in the central nervous system. J Neurol Sci. 2008;274:39–41.
Villar LM, García-Sánchez MI, Costa-Frossard L, Espiño M, Roldán E, Páramo D, et al. Immunological markers of optimal response to natalizumab in multiple sclerosis. Arch Neurol. 2012;69:191–7. The authors report that therapeutic responsiveness to natalizumab correlates with reductions in intrathecal IgM levels and in the frequency of CD8+ plasmablasts. Their data supports the contention that natalizumab regulates MS disease activity, at least in part, through its effects on B cells.
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15.
O'Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–303.
Gold R, Wolinsky JS. Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol Scand. 2011;124:75–84.
Freedman MS, Wolinsky JS, Wamil B, Confavreux C, Comi G, Kappos L, et al. Teriflunomide added to interferon-β in relapsing multiple sclerosis: a randomized phase II trial. Neurology. 2012;78:1877–85.
Siemasko KF, Chong AS, Williams JW, Bremer EG, Finnegan A. Regulation of B cell function by the immunosuppressive agent leflunomide. Transplantation. 1996;61:635–42.
Yan Y, Verbeken E, Yu L, Rutgeerts O, Goebels J, Segers C, et al. Effects of a short course of leflunomide on T-independent B-lymphocyte xenoreactivity and on susceptibility of xenografts to acute or chronic rejection. Transplantation. 2005;79:135–41. discussion 133–4.
Ramos-Barrón MA, Gómez-Alamillo C, Santiuste I, Agüeros C, Cosme LS, Benito A, et al. Leflunomide derivative FK778 inhibits production of antibodies in an experimental model of alloreactive T-B cell interaction. Exp Clin Transplant. 2009;7:218–24.
Merrill JE, Hanak S, Pu SF, Liang J, Dang C, Iglesias-Bregna D, et al. Teriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. J Neurol. 2009;256:89–103.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukemia: a randomized, open-label, phase 3 trial. Lancet. 2010;376:1164–74.
Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at 24 weeks. Arthritis Rheum. 2006;54:2793–806.
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88.
Rommer PS, Patejdl R, Winkelmann A, Benecke R, Zettl UK. Rituximab for secondary progressive multiple sclerosis: a case series. CNS Drugs. 2011;25:607–13.
Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–71.
Piccio L, Naismith RT, Trinkaus K, Klein RS, Parks BJ, Lyons JA, et al. Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch Neurol. 2010;67:707–14.
Petereit HF, Moeller-Hartmann W, Reske D, Rubbert A. Rituximab in a patient with multiple sclerosis—effect on B cells, plasma cells, and intrathecal IgG synthesis. Acta Neurol Scand. 2008;117:399–403.
Monson NL, Cravens P, Hussain R, Harp CT, Cummings M, de Pilar Martin M, et al. Rituximab therapy reduces organ-specific T cell responses and ameliorates experimental autoimmune encephalomyelitis. PLoS One. 2011;6:e17103.
Kappos L, Li D, Calabresi PA, O'Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomized, placebo-controlled, multicenter trial. Lancet. 2011;378:1779–87.
Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. Impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302.
Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–59.
Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 2008;58:61–72.
Dall'Era M, Chakravarty E, Wallace D, Genovese M, Weisman M, Kavanaugh A, et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 2007;56:4142–50.
Visser LH, Beekman R, Tijssen CC, Uitdehaag BM, Lee ML, Movig KL, et al. A randomized, double-blind, placebo-controlled pilot study of i.v. immune globulins in combination with i.v. methylprednisolone in the treatment of relapses in patients with MS. Mult Scler. 2004;10:89–91.
Otsuki T, Yamada O, Yata K, Sakaguchi H, Kurebayashi J, Yawata Y, et al. Expression and production of interleukin 10 in human myeloma cell lines. Br J Haematol. 2000;111:835–42.
Matthes T, Werner-Favre C, Zubler RH. Cytokine expression and regulation of human plasma cells: disappearance of interleukin-10 and persistence of transforming growth factor-beta 1. Eur J Immunol. 1995;25:508–12.
Conflict of Interest
Tiffany J. Braley declares that she has no conflict of interest.
Benjamin M. Segal has served as a consultant for Biogen Idec and Bristol-Myers Squibb, has received grant support from Teva Pharmaceuticals and Biogen Idec, and has had travel/accommodations expenses covered/reimbursed by Biogen Idec and Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Braley, T.J., Segal, B.M. B-Cell Targeting Agents in the Treatment of Multiple Sclerosis. Curr Treat Options Neurol 15, 259–269 (2013). https://doi.org/10.1007/s11940-013-0232-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-013-0232-y